Deck 12: Solids and Modern Materials
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 12: Solids and Modern Materials
1
Identify the type of solid for ice.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
molecular solid
2
Identify the type of solid for AgCl.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
ionic solid
3
Identify two-dimensional graphite.
A) buckyball
B) diamond
C) graphene
D) fullerene
E) charcoal
A) buckyball
B) diamond
C) graphene
D) fullerene
E) charcoal
graphene
4
Identify the characteristics of a face-centered cubic cell.
A) edge length = 2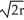 , 80% packing efficiency
, 80% packing efficiency
B) edge length = 2r, 52% packing efficiency
C) edge length = 4r/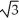 , 68% packing efficiency
, 68% packing efficiency
D) edge length = 2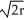 , 74% packing efficiency
, 74% packing efficiency
E) edge length = 2r, 60% packing efficiency
A) edge length = 2
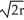 , 80% packing efficiency
, 80% packing efficiencyB) edge length = 2r, 52% packing efficiency
C) edge length = 4r/
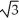 , 68% packing efficiency
, 68% packing efficiencyD) edge length = 2
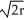 , 74% packing efficiency
, 74% packing efficiencyE) edge length = 2r, 60% packing efficiency

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the type of solid for diamond.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the characteristics of a simple cubic cell.
A) edge length = 2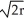 , 80% packing efficiency
, 80% packing efficiency
B) edge length = 2r, 52% packing efficiency
C) edge length = 4r/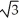 , 68% packing efficiency
, 68% packing efficiency
D) edge length = 2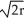 , 74% packing efficiency
, 74% packing efficiency
E) edge length = 2r, 60% packing efficiency
A) edge length = 2
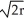 , 80% packing efficiency
, 80% packing efficiencyB) edge length = 2r, 52% packing efficiency
C) edge length = 4r/
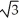 , 68% packing efficiency
, 68% packing efficiencyD) edge length = 2
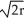 , 74% packing efficiency
, 74% packing efficiencyE) edge length = 2r, 60% packing efficiency

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the characteristics of a body-centered cubic cell.
A) edge length = 2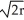 , 80% packing efficiency
, 80% packing efficiency
B) edge length = 2r, 52% packing efficiency
C) edge length = 4r/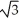 , 68% packing efficiency
, 68% packing efficiency
D) edge length = 2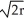 , 74% packing efficiency
, 74% packing efficiency
E) edge length = 2r, 60% packing efficiency
A) edge length = 2
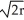 , 80% packing efficiency
, 80% packing efficiencyB) edge length = 2r, 52% packing efficiency
C) edge length = 4r/
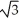 , 68% packing efficiency
, 68% packing efficiencyD) edge length = 2
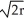 , 74% packing efficiency
, 74% packing efficiencyE) edge length = 2r, 60% packing efficiency

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
When two waves interact with the crests of one aligning with the troughs of the other is called
A) complimentary interference.
B) destructive interference.
C) opposing interference.
D) constructive interference.
E) alignment interference.
A) complimentary interference.
B) destructive interference.
C) opposing interference.
D) constructive interference.
E) alignment interference.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the type of solid for argon.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the type of solid for gold.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the thinnest known material.
A) buckyball
B) diamond
C) graphene
D) fullerene
E) charcoal
A) buckyball
B) diamond
C) graphene
D) fullerene
E) charcoal

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
To obtain the best chocolate,the chocolate is
A) cooled quickly.
B) heated quickly.
C) heated and cooled under controlled conditions.
D) dropped into ice water.
E) not monitored with temperature.
A) cooled quickly.
B) heated quickly.
C) heated and cooled under controlled conditions.
D) dropped into ice water.
E) not monitored with temperature.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
When an X-ray beam of l = 131.5 pm is incident on the surface of a copper crystal,it produces a maximum diffraction at an angle of q = 25.25 degrees.Assuming n = 1,calculate the separation between layers of copper atoms in the crystal.
A) 5.208 pm
B) 263.0 pm
C) 2.604 pm
D) 308.3 pm
E) 154.1 pm
A) 5.208 pm
B) 263.0 pm
C) 2.604 pm
D) 308.3 pm
E) 154.1 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
Define blooming with chocolate.
A) mixed unevenly
B) shatters easily
C) melting unevenly
D) white residue on the surface of chocolate
E) black residue on the surface of chocolate
A) mixed unevenly
B) shatters easily
C) melting unevenly
D) white residue on the surface of chocolate
E) black residue on the surface of chocolate

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the technique that determines the arrangement of atoms and measures the distance between them.
A) x-ray diffraction
B) ultraviolet
C) nuclear magnetic resonance
D) infrared
E) atomic absorption
A) x-ray diffraction
B) ultraviolet
C) nuclear magnetic resonance
D) infrared
E) atomic absorption

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
When two waves interact with their crests and troughs in alignment is called
A) complimentary interference.
B) destructive interference.
C) opposing interference.
D) constructive interference.
E) alignment interference.
A) complimentary interference.
B) destructive interference.
C) opposing interference.
D) constructive interference.
E) alignment interference.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 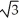 .
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
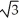 .
.A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
A metal crystallizes in a face centered cubic structure and has a density of 11.9 g/cm3.If the radius of the metal atom is 138 pm,what is the identity of the metal?
A) At
B) Pd
C) Mn
D) Fe
E) Cr
A) At
B) Pd
C) Mn
D) Fe
E) Cr

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
Determine the radius of an Al atom (in pm)if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 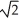 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
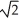 r.
r.A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
The best chocolate has a melting point of ________ °C.
A) 27.3
B) 17.3
C) 36.3
D) 33.8
E) 25.5
A) 27.3
B) 17.3
C) 36.3
D) 33.8
E) 25.5

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the compound that has the zinc blende structure.
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the formula for silica.
A) SiO4
B) SiO3
C) Si2O3
D) SiO2
E) Si3O6
A) SiO4
B) SiO3
C) Si2O3
D) SiO2
E) Si3O6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
An allotrope of carbon that exists as interconnected rings that assume a cylindrical shape is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
An allotrope of carbon that exists as face centered cubic structure is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the type of interaction between atoms in a nonbonding atomic solid.
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
An allotrope of carbon that consists of flat sheets of carbon atoms covalently bonded together as interconnected hexagonal rings is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
Identify a use for ceramics.
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the compound that has the same structure as calcium sulfide.
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the type of interaction between atoms in a networking atomic solid.
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces
A) polar bonding
B) ionic bonding
C) covalent bonding
D) hydrogen bonding
E) weak dispersion forces

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the largest unreinforced concrete dome in existence.
A) Pantheon
B) Hagia Sophia
C) Orbetello aircraft hangar
D) Ream's turtle
E) Turin's exposition hall
A) Pantheon
B) Hagia Sophia
C) Orbetello aircraft hangar
D) Ream's turtle
E) Turin's exposition hall

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
Identify an element that is NOT in silicate ceramics.
A) oxygen
B) silicon
C) aluminum
D) carbon
E) hydrogen
A) oxygen
B) silicon
C) aluminum
D) carbon
E) hydrogen

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the compound that has the same structure as sodium chloride.
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
Identify a compound that is NOT in cement.
A) iron(III) oxide
B) silica
C) gypsum
D) limestone
E) sodium carbonate
A) iron(III) oxide
B) silica
C) gypsum
D) limestone
E) sodium carbonate

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
An allotrope of carbon that exists in the shape of a ball containing 36 to 100 carbons is called
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.
A) an octahedral.
B) a nanotube.
C) a diamond.
D) a fullerene.
E) graphite.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
Identify an element that is NOT in nonoxide ceramics.
A) nitrogen
B) silicon
C) aluminum
D) boron
E) carbon
A) nitrogen
B) silicon
C) aluminum
D) boron
E) carbon

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the shape for SiO4.
A) tetrahedral
B) trigonal bipyramidal
C) trigonal planar
D) octahedral
E) trigonal pyramidal
A) tetrahedral
B) trigonal bipyramidal
C) trigonal planar
D) octahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
Identify a use for cement.
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the compound that has fluorite structure.
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water
A) silver iodide
B) barium chloride
C) potassium bromide
D) cesium chloride
E) water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
Geologists estimate that ________ percent of the Earth's crust is composed of silicates.
A) 80
B) 75
C) 85
D) 90
E) 70
A) 80
B) 75
C) 85
D) 90
E) 70

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
Identify an element that is NOT in oxide ceramics.
A) oxygen
B) silicon
C) aluminum
D) magnesium
A) oxygen
B) silicon
C) aluminum
D) magnesium

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
Identify for the rhombohedral unit cell.
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
A common use of polyethylene terephthalate is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following represent the addition polymer formed from the compound below? CH2=CH-CH3
A)
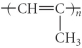
B)
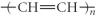
C)
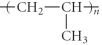
D)
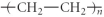
E)
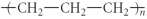
A)
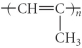
B)
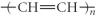
C)
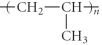
D)
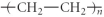
E)
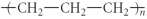

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the element with the largest band gap.
A) lead
B) silicon
C) germanium
D) carbon
E) tin
A) lead
B) silicon
C) germanium
D) carbon
E) tin

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the element with the smallest band gap.
A) lead
B) silicon
C) germanium
D) carbon
E) tin
A) lead
B) silicon
C) germanium
D) carbon
E) tin

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
Identify a use for glass.
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks
A) beakers
B) bridges
C) furniture
D) wooden decks
E) bricks

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
A common use of nylon 6,6 is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the copolymer.
A) nylon 6,6
B) polyvinyl chloride
C) polyethylene
D) polypropylene
E) polystyrene
A) nylon 6,6
B) polyvinyl chloride
C) polyethylene
D) polypropylene
E) polystyrene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the element that is the best insulator.
A) lead
B) silicon
C) germanium
D) carbon
E) tin
A) lead
B) silicon
C) germanium
D) carbon
E) tin

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following represent the addition polymer formed from the compound below? CH2=CHCl
A)
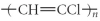
B)
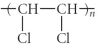
C)
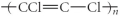
D)
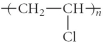
E)
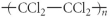
A)
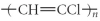
B)
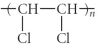
C)
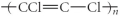
D)
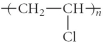
E)
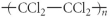

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
Identify for the hexagonal unit cell.
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
A common use of polystyrene is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
A common use of polyethylene is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
Identify a compound that is NOT used in the making of any glass.
A) SiO2
B) Na2O
C) CaO
D) NaCl
E) B2O3
A) SiO2
B) Na2O
C) CaO
D) NaCl
E) B2O3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
A common use of polyurethane is
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.
A) tire cord.
B) fishing line.
C) foam cups.
D) spray-on insulation.
E) films.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
Identify an element that is added to glass to give it a higher index of refraction and a more brilliant appearance than soda-lime glass.
A) calcium
B) sodium
C) lead
D) boron
E) potassium
A) calcium
B) sodium
C) lead
D) boron
E) potassium

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
A common use of polyvinyl chloride is
A) plumbing.
B) plastic wrap.
C) plastic bottles.
D) styrofoam.
E) nylon.
A) plumbing.
B) plastic wrap.
C) plastic bottles.
D) styrofoam.
E) nylon.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the condensation polymer.
A) polyurethane
B) polypropylene
C) polyvinyl chloride
D) polyethylene
E) polystyrene
A) polyurethane
B) polypropylene
C) polyvinyl chloride
D) polyethylene
E) polystyrene

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
Identify what Kevlar is NOT used for.
A) helmets
B) bullet-proof vests
C) roads
D) brake pads
E) suspension bridge cables
A) helmets
B) bullet-proof vests
C) roads
D) brake pads
E) suspension bridge cables

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the addition polymer.
A) polyurethane
B) polypropylene
C) polyethylene terephthalate
D) nylon 6,6
E) polyester
A) polyurethane
B) polypropylene
C) polyethylene terephthalate
D) nylon 6,6
E) polyester

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
Which type of bonding does Sr form upon solidification?
A) covalent network
B) ionic
C) metallic
D) molecular
A) covalent network
B) ionic
C) metallic
D) molecular

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
Identify for the triclinic unit cell.
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c
A) a ≠ b = c
B) a = b ≠ c
C) a ≠ b ≠ c
D) a = b = c

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Rubidium crystallizes in a body-centered cubic structure.What is the coordination number of each atom?
A) 2
B) 6
C) 8
D) 12
E) 16
A) 2
B) 6
C) 8
D) 12
E) 16

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
Cesium has a radius of 272 pm and crystallizes in a body-centered cubic structure.What is the edge length of the unit cell?
A) 314 pm
B) 385 pm
C) 544 pm
D) 628 pm
A) 314 pm
B) 385 pm
C) 544 pm
D) 628 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
Nickel has a face-centered cubic structure and has a density of 8.90 g/cm3.What is its atomic radius?
A) 125 pm
B) 249 pm
C) 353 pm
D) 997 pm
A) 125 pm
B) 249 pm
C) 353 pm
D) 997 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
How many H- ions are around each Na+ ion in NaH,which has a cubic unit cell with H- ions on each corner and each face?
A) 1
B) 4
C) 6
D) 8
A) 1
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following forms an ionic solid?
A) Co
B) C4H9NH2
C) NH4NO3
D) SO3
A) Co
B) C4H9NH2
C) NH4NO3
D) SO3

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
Give the edge length in terms of r for a simple cubic cell.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following forms a molecular solid?
A) NH4NO3
B) C10H22
C) C, diamond
D) silver
A) NH4NO3
B) C10H22
C) C, diamond
D) silver

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is considered an atomic solid?
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is considered a nonbonding atomic solid?
A) Ne
B) Cu
C) I2
D) Ca
E) K
A) Ne
B) Cu
C) I2
D) Ca
E) K

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is considered a molecular solid?
A) Ag
B) NH4NO3
C) H2O
D) Kr
E) None of these is a molecular solid.
A) Ag
B) NH4NO3
C) H2O
D) Kr
E) None of these is a molecular solid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the unit cell that has α = β = γ = 90°.
A) cubic
B) rhombohedral
C) tetrahedral
D) monoclinic
A) cubic
B) rhombohedral
C) tetrahedral
D) monoclinic

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is considered an ionic solid?
A) (NH4)2CO3
B) CCl4
C) OCBr2
D) XeF4
E) None of these is an ionic solid.
A) (NH4)2CO3
B) CCl4
C) OCBr2
D) XeF4
E) None of these is an ionic solid.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
NaCl crystallizes in a cubic unit cell with Cl- ions on each corner and each face.How many Na+ and Cl- ions are in each unit cell of NaCl?
A) 1 Na+ ion and 1 Cl- ion
B) 2 Na+ ions and 2 Cl- ions
C) 4 Na+ ions and 4 Cl- ions
D) 8 Na+ ions and 8 Cl- ions
A) 1 Na+ ion and 1 Cl- ion
B) 2 Na+ ions and 2 Cl- ions
C) 4 Na+ ions and 4 Cl- ions
D) 8 Na+ ions and 8 Cl- ions

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
What is the edge length of a face-centered cubic unit cell made up of atoms having a radius of 125 pm?
A) 177 pm
B) 354 pm
C) 500 pm
D) 1000 pm
E) 800 pm
A) 177 pm
B) 354 pm
C) 500 pm
D) 1000 pm
E) 800 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following substances should have the highest melting point?
A) Fe
B) Ne
C) Xe
D) N2
E) CO
A) Fe
B) Ne
C) Xe
D) N2
E) CO

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following substances should have the highest melting point?
A) CO2
B) CH4
C) Ar
D) I2
E) MgO
A) CO2
B) CH4
C) Ar
D) I2
E) MgO

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
79
Gold crystallizes in a face-centered cubic structure.What is the edge length of the unit cell if the atomic radius of gold is 144 pm?
A) 204 pm
B) 288 pm
C) 333 pm
D) 407 pm
A) 204 pm
B) 288 pm
C) 333 pm
D) 407 pm

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
80
Give the coordination number for a face-centered cubic cell.
A) 2
B) 8
C) 12
D) 10
E) 6
A) 2
B) 8
C) 12
D) 10
E) 6

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck


