Deck 25: Transition Metals and Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 25: Transition Metals and Coordination Compounds
1
Identify the structure that fac-mer isomerism can occur in.
A) MA4B2
B) MA5B
C) MAB
D) MA3B3
E) MAB2
A) MA4B2
B) MA5B
C) MAB
D) MA3B3
E) MAB2
MA3B3
2
Name the following: [Fe(CO)6][Ag(CN)2]2
A) iron(III) hexacarbonylsilverdicyanide
B) hexacarbonyliron(II) dicyanoargentate
C) iron(II) carbonyl silvercyanide
D) hexacarbonyliron(III) dicyanosilvate
E) iron(II) hexacarbonyl silvercyanide
A) iron(III) hexacarbonylsilverdicyanide
B) hexacarbonyliron(II) dicyanoargentate
C) iron(II) carbonyl silvercyanide
D) hexacarbonyliron(III) dicyanosilvate
E) iron(II) hexacarbonyl silvercyanide
hexacarbonyliron(II) dicyanoargentate
3
Identify the geometry of the complex ion if the hybridization is d2sp3.
A) square planar
B) octahedral
C) tetrahedral
D) linear
E) trigonal planar
A) square planar
B) octahedral
C) tetrahedral
D) linear
E) trigonal planar
octahedral
4
Choose the electron configuration for Fe3+.
A) [Ar]4s23d3
B) [Ar]3d5
C) [Ar]4s23d9
D) [Ar]4s13d4
E) [Ar]4s23d6
A) [Ar]4s23d3
B) [Ar]3d5
C) [Ar]4s23d9
D) [Ar]4s13d4
E) [Ar]4s23d6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the structure that cis-trans isomerism can occur in.
A) MA4B2
B) MA5B
C) MAB
D) MA3B3
E) MAB2
A) MA4B2
B) MA5B
C) MAB
D) MA3B3
E) MAB2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the chemical formula for the compound,tetracarbonylplatinum(IV)chloride.
A) [Pt(CO)4]Cl2
B) [Pt(CO)4]Cl4
C) [Pt(CO)4Cl4]2-
D) [PtCl4](CO)4
E) [Pt(CO)4Cl4]4-
A) [Pt(CO)4]Cl2
B) [Pt(CO)4]Cl4
C) [Pt(CO)4Cl4]2-
D) [PtCl4](CO)4
E) [Pt(CO)4Cl4]4-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Identify the ion that is responsible for the red in garnet and the yellow-green of peridot.
A) Cr2+
B) Cu+
C) Cu2+
D) Cr3+
E) Fe2+
A) Cr2+
B) Cu+
C) Cu2+
D) Cr3+
E) Fe2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the ion that is responsible for the green color in emeralds.
A) Cr3+
B) Cr4+
C) Cr5+
D) Cr6+
E) Cr7+
A) Cr3+
B) Cr4+
C) Cr5+
D) Cr6+
E) Cr7+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the geometry of the complex ion if the hybridization is dsp2.
A) square planar
B) octahedral
C) tetrahedral
D) linear
E) trigonal planar
A) square planar
B) octahedral
C) tetrahedral
D) linear
E) trigonal planar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
Name the following: Fe[AlF6]
A) ironaluminumhexafluoride
B) iron(II) hexafluoroaluminum
C) iron(III) hexafluoroaluminate
D) iron(I) aluminumhexafluoride
E) aluminumhexafluoroferrate
A) ironaluminumhexafluoride
B) iron(II) hexafluoroaluminum
C) iron(III) hexafluoroaluminate
D) iron(I) aluminumhexafluoride
E) aluminumhexafluoroferrate

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the ion that is responsible for the red color of rubies.
A) Cr3+
B) Cr4+
C) Cr5+
D) Cr6+
E) Cr7+
A) Cr3+
B) Cr4+
C) Cr5+
D) Cr6+
E) Cr7+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
Determine the chemical formula for the compound,diaquadicarbonylzinc tetrabromopalladate(IV).
A) [PdZn(H2O)2(CO)2]Br4
B) [Zn(H2O)2(CO)2]2[PdBr4]
C) [Pd(H2O)2][Zn(CO)2]Br4
D) [Pd(H2O)2]2[Zn(CO)2]3Br4
E) [Zn(H2O)2(CO)2][PdBr4]
A) [PdZn(H2O)2(CO)2]Br4
B) [Zn(H2O)2(CO)2]2[PdBr4]
C) [Pd(H2O)2][Zn(CO)2]Br4
D) [Pd(H2O)2]2[Zn(CO)2]3Br4
E) [Zn(H2O)2(CO)2][PdBr4]

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
Identify the geometry of [Zn(NH3)4]+2.
A) tetrahedral
B) octahedral
C) linear
D) square planar
E) trigonal planar
A) tetrahedral
B) octahedral
C) linear
D) square planar
E) trigonal planar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the ion that is responsible for the blue of turquoise.
A) Cr2+
B) Cu+
C) Cu2+
D) Cr3+
E) Fe2+
A) Cr2+
B) Cu+
C) Cu2+
D) Cr3+
E) Fe2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the isomers that have ligands with different spatial arrangements about the metal ions.
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) structural isomers
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) structural isomers

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following complex ions absorbs light of the longest wavelength?
A) [Cr(CN)6]3-
B) [CrCl6]3-
C) [Cr(en)3]3+
D) [Cr(NH3)6]3+
E) [Cr(NO2)6]3-
A) [Cr(CN)6]3-
B) [CrCl6]3-
C) [Cr(en)3]3+
D) [Cr(NH3)6]3+
E) [Cr(NO2)6]3-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Determine the chemical formula for the compound,potassiumtrisethylenediamine chromate(III).
A) K3[Cr(NH2CH2CH2NH2)3]
B) [K(NH2CH2CH2NH2)3]Cr3+
C) [KCr(NH2CH2CH2NH2)3]3+
D) K2[Cr(NH2CH2CH2NH2)4]
E) [Cr(NH2CH2CH2NH2)2]K2
A) K3[Cr(NH2CH2CH2NH2)3]
B) [K(NH2CH2CH2NH2)3]Cr3+
C) [KCr(NH2CH2CH2NH2)3]3+
D) K2[Cr(NH2CH2CH2NH2)4]
E) [Cr(NH2CH2CH2NH2)2]K2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the isomers that occurs when a coordinated ligand exchanges places with the uncoordinated counterion.
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) stereoisomers
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) stereoisomers

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Name the following: [Ni(NH3)3(H2O)3]Cl2
A) nickel(II) trihydrotriamminechloride
B) nickel(I) triamminetrihydrochloride
C) dichloronickel(II) triamminetrihydride
D) nickel(III) chloride
E) triamminetriaquanickel(II) chloride
A) nickel(II) trihydrotriamminechloride
B) nickel(I) triamminetrihydrochloride
C) dichloronickel(II) triamminetrihydride
D) nickel(III) chloride
E) triamminetriaquanickel(II) chloride

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the isomers that have ligands which coordinates to metal in different ways.
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) stereoisomers
A) linkage isomers
B) geometric isomers
C) coordination isomers
D) optical isomers
E) stereoisomers

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
What is the highest possible oxidation state for cobalt?
A) +1
B) +7
C) +6
D) +4
E) +5
A) +1
B) +7
C) +6
D) +4
E) +5

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the compound that is used to treat victims of heavy metal poisoning.
A) KCN
B) CO
C) EDTA
D) carbonic anhydrase
E) cisplatin
A) KCN
B) CO
C) EDTA
D) carbonic anhydrase
E) cisplatin

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
How many unpaired electrons would you expect for the complex ion: [FeCl6]4-?
A) 2
B) 6
C) 4
D) 3
E) 0
A) 2
B) 6
C) 4
D) 3
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the compound that an effective anticancer agent.
A) KCN
B) CO
C) EDTA
D) carbonic anhydrase
E) cisplatin
A) KCN
B) CO
C) EDTA
D) carbonic anhydrase
E) cisplatin

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the electron configuration for Nb3+.
A) [Kr]5s24d2
B) [Kr]5s2
C) [Kr]4d2
D) [Ar]4s23d104p6
E) [Kr]5s24d4
A) [Kr]5s24d2
B) [Kr]5s2
C) [Kr]4d2
D) [Ar]4s23d104p6
E) [Kr]5s24d4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
How many d electrons are in W O42-?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
How many unpaired electrons would you expect for the complex ion: [Cr(CN)6]4-?
A) 0
B) 5
C) 3
D) 1
E) 2
A) 0
B) 5
C) 3
D) 1
E) 2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Choose the electron configuration for Cr3+.
A) [Ne]3s23p6
B) [Ar]4s13d2
C) [Ar]4s23d1
D) [Ar]4s23d7
E) [Ar]3d3
A) [Ne]3s23p6
B) [Ar]4s13d2
C) [Ar]4s23d1
D) [Ar]4s23d7
E) [Ar]3d3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the electron configuration for V3+.
A) [Ar]3d2
B) [Ar]4s23d2
C) [Ar]4s23d4
D) [Ar]4s2
E) [Ne]3s23p6
A) [Ar]3d2
B) [Ar]4s23d2
C) [Ar]4s23d4
D) [Ar]4s2
E) [Ne]3s23p6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the geometry around the Fe2+ ion in the hemoglobin complex.
A) octahedral
B) tetrahedral
C) pentadral
D) hexadral
E) heptadral
A) octahedral
B) tetrahedral
C) pentadral
D) hexadral
E) heptadral

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following can function as a bidentate ligand?
A) NO +
B) OH -
C) S CN -
D) H2NCH2CO2-
A) NO +
B) OH -
C) S CN -
D) H2NCH2CO2-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
What is the ground-state electron configuration for Cr3+ (Z = 24)?
A) [Ar] 4s2 3d7
B) [Ar] 4s2 3d1
C) [Ar] 3d3
D) [Ar] 4s1 3d2
A) [Ar] 4s2 3d7
B) [Ar] 4s2 3d1
C) [Ar] 3d3
D) [Ar] 4s1 3d2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following complex ions absorbs light of the shortest wavelength?
A) [FeCl6]3-
B) [Fe(OH)6]3-
C) [Fe(CN)6]3-
D) [Fe(H2O)6]3+
E) [FeI6]3-
A) [FeCl6]3-
B) [Fe(OH)6]3-
C) [Fe(CN)6]3-
D) [Fe(H2O)6]3+
E) [FeI6]3-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
What is the ground-state electron configuration for the element copper (Z = 29)?
A) [Ne] 4s2 3d9
B) [Ar] 4s2 3d9
C) [Ar] 4s1 3d10
D) [Ar] 3d9
A) [Ne] 4s2 3d9
B) [Ar] 4s2 3d9
C) [Ar] 4s1 3d10
D) [Ar] 3d9

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
The complex ion,[Ni(NH3)6]2+,has a maximum absorption near 580 nm.Calculate the crystal field splitting energy (in kJ/mol)for this ion.
A) 114 kJ/mol
B) 292 kJ/mol
C) 343 kJ/mol
D) 206 kJ/mol
E) 485 kJ/mol
A) 114 kJ/mol
B) 292 kJ/mol
C) 343 kJ/mol
D) 206 kJ/mol
E) 485 kJ/mol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
How many unpaired electrons would you expect for the complex ion: [Co(OH)6]3-?
A) 1
B) 2
C) 4
D) 0
E) 3
A) 1
B) 2
C) 4
D) 0
E) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
Identify the transition metal that is the coordinated metal in chlorophyll.
A) magnesium
B) manganese
C) selenium
D) iron
E) silver
A) magnesium
B) manganese
C) selenium
D) iron
E) silver

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
How many unpaired electrons would you expect for the complex ion: [Fe(NH3)6]2+?
A) 5
B) 2
C) 6
D) 4
E) 0
A) 5
B) 2
C) 6
D) 4
E) 0

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following species has the electron configuration [Ar]3d6?
A) Cr
B) Fe3+
C) Co3+
D) Ni3+
A) Cr
B) Fe3+
C) Co3+
D) Ni3+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Choose the electron configuration for Ru7+.
A) [Kr]5s14d1
B) [Kr]5s24d3
C) [Kr]5s24d1
D) [Kr]
E) [Kr]5s24d6
A) [Kr]5s14d1
B) [Kr]5s24d3
C) [Kr]5s24d1
D) [Kr]
E) [Kr]5s24d6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following pairs of coordination compounds or complex ions are examples of coordination isomers?
A) [Fe(NH3)5NO2]2+ and [Fe(NH3)5ONO]2+
B) [Fe(NH3)2(H2O)4]Cl2 and [Fe(NH3)2(H2O)4]Br2
C) [Fe(NH3)2(H2O)4]Br2 and [Fe(NH3)4(H2O)2]Br2
D) [Cu(CO)5I]F and [Cu(CO)5CF]I
E) [MnCl3Br]2- and [MnClBr3]2-
A) [Fe(NH3)5NO2]2+ and [Fe(NH3)5ONO]2+
B) [Fe(NH3)2(H2O)4]Cl2 and [Fe(NH3)2(H2O)4]Br2
C) [Fe(NH3)2(H2O)4]Br2 and [Fe(NH3)4(H2O)2]Br2
D) [Cu(CO)5I]F and [Cu(CO)5CF]I
E) [MnCl3Br]2- and [MnClBr3]2-

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following compounds can exhibit fac-mer isomerism?
A) [Cr(H2O)4Br2]+
B) [Fe(CO)5ONO]2+
C) [Fe(CO)3(NH3)3]3+
D) [Cu(CO)5Br]+
E) [V(NH3)4(H2O)2]2+
A) [Cr(H2O)4Br2]+
B) [Fe(CO)5ONO]2+
C) [Fe(CO)3(NH3)3]3+
D) [Cu(CO)5Br]+
E) [V(NH3)4(H2O)2]2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compounds can exhibit cis-trans isomerism?
A) [Cr(H2O)4Cl2]+
B) [Cu(CO)5Cl]+
C) [Co(CO)3(NH3)3]3+
D) [Co(CO)5ONO]2+
E) [Fe(NH3)6]2+
A) [Cr(H2O)4Cl2]+
B) [Cu(CO)5Cl]+
C) [Co(CO)3(NH3)3]3+
D) [Co(CO)5ONO]2+
E) [Fe(NH3)6]2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
What type of hybridization (according to valence bond theory)does Fe exhibit in the complex ion,[Fe(H2O)4(Br)2]+?
A) dsp3
B) sp3
C) d2sp2
D) d2sp3
E) d2sp
A) dsp3
B) sp3
C) d2sp2
D) d2sp3
E) d2sp

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the chemical formula for the compound,diamminetetraaquamanganese(II)bromide.
A) [Mn(NH3)2(H2O)4Br]
B) [Mn(NH3)2][(H2O)4Br]
C) [Mn(NH3)2(H2O)4]Br2
D) [Mn(H2O)4][(NH3)2Br]
E) [Mn(NH3)2(H2O)4]Br3
A) [Mn(NH3)2(H2O)4Br]
B) [Mn(NH3)2][(H2O)4Br]
C) [Mn(NH3)2(H2O)4]Br2
D) [Mn(H2O)4][(NH3)2Br]
E) [Mn(NH3)2(H2O)4]Br3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
Name the following: [Pt(H2O)4Br2]F2
A) tetraaquadibromoplatinum(IV) fluoride
B) tetraaquadibromoplatinate difluoride
C) platinum(II) fluoride
D) platinum(III) tetraaquadibromofluoride
E) platinum(II) dibromotetrahydride difluoride
A) tetraaquadibromoplatinum(IV) fluoride
B) tetraaquadibromoplatinate difluoride
C) platinum(II) fluoride
D) platinum(III) tetraaquadibromofluoride
E) platinum(II) dibromotetrahydride difluoride

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
Identify the geometry of [Ag(NH3)2]+.
A) tetrahedral
B) square planar
C) linear
D) bent
E) trigonal pyramidal
A) tetrahedral
B) square planar
C) linear
D) bent
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following species is diamagnetic?
A) an isolated, gas-phase Mn2+ ion
B) a high-spin octahedral Fe2+ complex
C) an isolated, gas-phase Cu2+ ion
D) a low-spin octahedral Co3+ complex
A) an isolated, gas-phase Mn2+ ion
B) a high-spin octahedral Fe2+ complex
C) an isolated, gas-phase Cu2+ ion
D) a low-spin octahedral Co3+ complex

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the chemical formula for the compound,hexammineiron(III)hexacyanoferrate(III).
A) [Fe2(NH3)6(CN)6]3+
B) [Fe(NH3)6][Fe(CN)6]2
C) [Fe(NH3)6]3[Fe(CN)6]2
D) [Fe(NH3)6][Fe(CN)6]
E) [Fe2(NH3)3(CN)3]6+
A) [Fe2(NH3)6(CN)6]3+
B) [Fe(NH3)6][Fe(CN)6]2
C) [Fe(NH3)6]3[Fe(CN)6]2
D) [Fe(NH3)6][Fe(CN)6]
E) [Fe2(NH3)3(CN)3]6+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
Choose the bidentate ligand from the substances below.
A) ethylenediamine
B) EDTA
C) chloride ion
D) ammonia
E) carbon monoxide
A) ethylenediamine
B) EDTA
C) chloride ion
D) ammonia
E) carbon monoxide

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the geometry of [PtCl4]2-.
A) tetrahedral
B) square planar
C) linear
D) octahedral
E) trigonal bipyramidal
A) tetrahedral
B) square planar
C) linear
D) octahedral
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Choose the polydentate ligand from the substances below.
A) oxalate ion
B) EDTA
C) nitrite ion
D) hydroxide ion
E) carbon monoxide
A) oxalate ion
B) EDTA
C) nitrite ion
D) hydroxide ion
E) carbon monoxide

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following can function as a chelating agent?
A) OH-
B) H2O
C) C2O42-
D) NH3
A) OH-
B) H2O
C) C2O42-
D) NH3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
How many moles of aqueous ions will be produced from the dissolution of 1.0 mole of Na3[FeCl6] in water?
A) 4
B) 10
C) 2
D) 9
E) 1
A) 4
B) 10
C) 2
D) 9
E) 1

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following pairs of coordination compounds or complex ions are examples of linkage isomers?
A) [Cu(NH3)5Br]Cl and [Cu(NH3)5Cl]Br
B) [Fe(NH3)2(H2O)4]Br2 and [Fe(NH3)4(H2O)2]Br2
C) [Mn(CO)5NO2]2+ and [Mn(CO)5ONO]2+
D) [Fe(NH3)2(H2O)4]Cl2 and [Fe(NH3)2(H2O)4]I2
E) [Ti(H2O)6]3+ and [Ti(NH3)6]3+
A) [Cu(NH3)5Br]Cl and [Cu(NH3)5Cl]Br
B) [Fe(NH3)2(H2O)4]Br2 and [Fe(NH3)4(H2O)2]Br2
C) [Mn(CO)5NO2]2+ and [Mn(CO)5ONO]2+
D) [Fe(NH3)2(H2O)4]Cl2 and [Fe(NH3)2(H2O)4]I2
E) [Ti(H2O)6]3+ and [Ti(NH3)6]3+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds can exhibit fac-mer isomerism?
A) [Cu(CO)5I]+
B) [Fe(H2O)3(CO)3]3+
C) [Ni(CO)5NO2]2+
D) [V(NH3)2(H2O)4]2+
E) [Cr(H2O)4Br2]+
A) [Cu(CO)5I]+
B) [Fe(H2O)3(CO)3]3+
C) [Ni(CO)5NO2]2+
D) [V(NH3)2(H2O)4]2+
E) [Cr(H2O)4Br2]+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds can exhibit cis-trans isomerism?
A) [Fe(CO)5NO2]2+
B) [Cu(CO)5Br]+
C) [MnClBr3]2-
D) [Mn(H2O)6]3+
E) [Ni(CO)2(NH3)2]2+
A) [Fe(CO)5NO2]2+
B) [Cu(CO)5Br]+
C) [MnClBr3]2-
D) [Mn(H2O)6]3+
E) [Ni(CO)2(NH3)2]2+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
Which ion would you expect to have the largest crystal field splitting Δ?
A) [Rh(CN)6]4-
B) [Rh(CN)6]3-
C) [Rh(H2O)6]2+
D) [Rh(H2O)6]3+
A) [Rh(CN)6]4-
B) [Rh(CN)6]3-
C) [Rh(H2O)6]2+
D) [Rh(H2O)6]3+

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
Name the following: [Fe(H2O)4F2]F
A) tetraaquadifluoroiron(II) fluoride
B) difluorotetrahydroironfluoride
C) difluorotetrahydroiron(II) fluoride
D) tetraaquadifluoroiron(III) fluoride
E) iron(II) difluorotetraquafluoride
A) tetraaquadifluoroiron(II) fluoride
B) difluorotetrahydroironfluoride
C) difluorotetrahydroiron(II) fluoride
D) tetraaquadifluoroiron(III) fluoride
E) iron(II) difluorotetraquafluoride

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the geometry of [Fe(H2O)6]3+.
A) tetrahedral
B) square pyramidal
C) linear
D) octahedral
E) trigonal planar
A) tetrahedral
B) square pyramidal
C) linear
D) octahedral
E) trigonal planar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Match between columns

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
Explain how EDTA is used to treat lead poisoning.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
What is the difference between a weak-field complex and a strong-field complex?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Why is +2 a common oxidation state for transition elements?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
What is a coordinate covalent bond?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
How many unpaired electrons would you expect for the complex ion: [Co(NH3 )6]4+?
A) 1
B) 0
C) 5
D) 2
E) 3
A) 1
B) 0
C) 5
D) 2
E) 3

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the transition metal that is used in hemoglobin synthesis in the human body.
A) potassium
B) zinc
C) copper
D) manganese
E) chromium
A) potassium
B) zinc
C) copper
D) manganese
E) chromium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Identify the transition metal that is used in fat and carbohydrate synthesis in the human body.
A) potassium
B) zinc
C) copper
D) manganese
E) chromium
A) potassium
B) zinc
C) copper
D) manganese
E) chromium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Match the following.
A)[Cr(NH3)3(CO)3]3+
B)[Ni(CN)2(OH)2]2-
C)[Zn(NH3)2Cl2]Br2 and [Zn(NH3)2Br2]Cl2
D)[Cr(en)3]3+
E)[Cu(SCN)4]3- and [Cu(NCS)4]3-
1)linkage
2)coordination
3)cis-trans
4)mer-fac
5)optical
A)[Cr(NH3)3(CO)3]3+
B)[Ni(CN)2(OH)2]2-
C)[Zn(NH3)2Cl2]Br2 and [Zn(NH3)2Br2]Cl2
D)[Cr(en)3]3+
E)[Cu(SCN)4]3- and [Cu(NCS)4]3-
1)linkage
2)coordination
3)cis-trans
4)mer-fac
5)optical

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
Identify the transition metal that is used in oxygen transport in the human body.
A) chromium
B) iron
C) sodium
D) copper
E) silver
A) chromium
B) iron
C) sodium
D) copper
E) silver

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
What type of geometry (according to valence bond theory)does V exhibit in the complex ion: [V(NH3)4]2+?
A) square bipyramidal
B) see-saw
C) trigonal pyramidal
D) bent
E) square planar
A) square bipyramidal
B) see-saw
C) trigonal pyramidal
D) bent
E) square planar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
What type of hybridization (according to valence bond theory)does Pt exhibit in the complex ion,[Pt(NH3)4]2+?
A) dsp3
B) sp
C) d2sp3
D) d2sp
E) dsp2
A) dsp3
B) sp
C) d2sp3
D) d2sp
E) dsp2

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
What is the Lanthanide contraction?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
What type of geometry (according to valence bond theory)does Co exhibit in the complex ion, 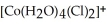 ?
?
A) linear
B) trigonal planar
C) square pyramidal
D) octahedral
E) tetrahedral
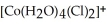 ?
?A) linear
B) trigonal planar
C) square pyramidal
D) octahedral
E) tetrahedral

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


