Deck 9: An Overview of the Most Common Elementary Steps
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 9: An Overview of the Most Common Elementary Steps
1
Which of the following can behave as an electrophile?
A)CH3O-
B)Cl-
C)HBr
D)CH3CH2CH2CH3
E)None of the above
A)CH3O-
B)Cl-
C)HBr
D)CH3CH2CH2CH3
E)None of the above
HBr
2
Which of the following is true of SN2 reactions?
A)They require two mechanism steps.
B)They involve two nucleophiles.
C)They do not require a leaving group.
D)They require one atom or group of atoms to be replaced by another atom or group of atoms.
E)They require two electron-poor species to react.
A)They require two mechanism steps.
B)They involve two nucleophiles.
C)They do not require a leaving group.
D)They require one atom or group of atoms to be replaced by another atom or group of atoms.
E)They require two electron-poor species to react.
They require one atom or group of atoms to be replaced by another atom or group of atoms.
3
Which of the following is not true about Lewis bases?
A)They donate electron density.
B)They often bear a negative charge.
C)They attack electron-poor sites.
D)They tend to be electron rich.
E)They tend to be electrophiles.
A)They donate electron density.
B)They often bear a negative charge.
C)They attack electron-poor sites.
D)They tend to be electron rich.
E)They tend to be electrophiles.
They tend to be electrophiles.
4
Which of the following is a coordination step? 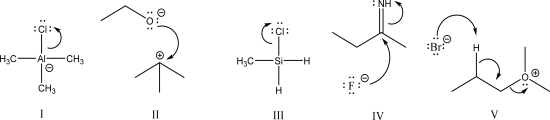
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is an E2 step? 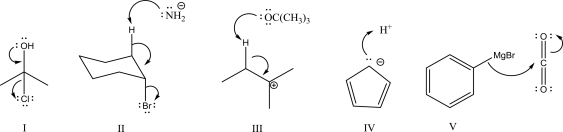
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is not true about a nucleophile elimination step?
A)A leaving group leaves.
B)A multiple bond is formed.
C)Only one product is formed.
D)Electrons move from electron-rich to electron-poor parts of a molecule.
E)Two mechanism arrows are used.
A)A leaving group leaves.
B)A multiple bond is formed.
C)Only one product is formed.
D)Electrons move from electron-rich to electron-poor parts of a molecule.
E)Two mechanism arrows are used.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
What type of mechanism step is shown below? 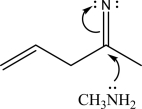
A)SN2
B)Nucleophilic addition
C)E2
D)Electrophile elimination
E)Nucleophile elimination

A)SN2
B)Nucleophilic addition
C)E2
D)Electrophile elimination
E)Nucleophile elimination

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is an SN2 step? 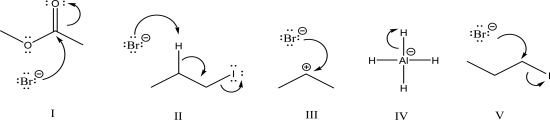
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a coordination step? 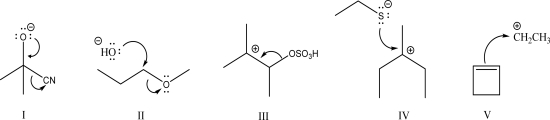
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
What is the most likely mechanism for the following reaction? 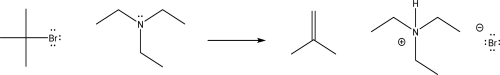
A)SN2
B)E2
C)Electrophilic addition
D)1,2 methyl shift
E)Nucleophilic addition

A)SN2
B)E2
C)Electrophilic addition
D)1,2 methyl shift
E)Nucleophilic addition

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true about Lewis acids?
A)They accept electrons.
B)They often have a negative charge.
C)They tend to be electron rich.
D)They attract electron-poor sites.
E)They only attract protons.
A)They accept electrons.
B)They often have a negative charge.
C)They tend to be electron rich.
D)They attract electron-poor sites.
E)They only attract protons.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an acceptable mechanism step? 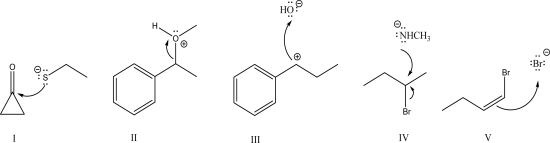
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is considered heterolysis? 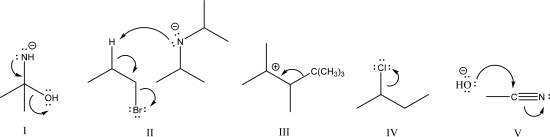
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not true about Grignard reagents?
A)They include a metal directly bonded to a carbon.
B)They tend to be electrophiles.
C)They are useful for forming new carbon-carbon bonds.
D)They consist of a highly polar bond.
E)They are organometallic compounds.
A)They include a metal directly bonded to a carbon.
B)They tend to be electrophiles.
C)They are useful for forming new carbon-carbon bonds.
D)They consist of a highly polar bond.
E)They are organometallic compounds.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
The following mechanism step is ________. 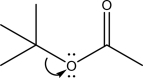
A)Heterolysis
B)Coordination
C)Nucleophile elimination
D)E2
E)Electrophile elimination

A)Heterolysis
B)Coordination
C)Nucleophile elimination
D)E2
E)Electrophile elimination

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is true of curved arrow notation showing reaction mechanisms?
A)Arrows begin at the electrophile.
B)Arrows point to nucleophiles.
C)Arrows show electrons flowing from nucleophiles to electrophiles.
D)Only one arrow is required.
E)Arrows never begin at the middle of a multiple bond.
A)Arrows begin at the electrophile.
B)Arrows point to nucleophiles.
C)Arrows show electrons flowing from nucleophiles to electrophiles.
D)Only one arrow is required.
E)Arrows never begin at the middle of a multiple bond.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is an acceptable mechanism step? 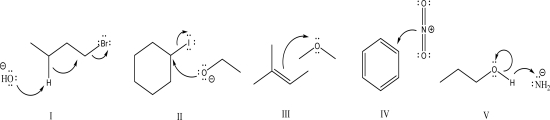
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
What type of mechanism step is shown below? 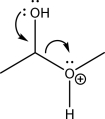
A)SN2
B)Nucleophilic addition
C)Electrophilic addition
D)Electrophile elimination
E)Nucleophile elimination

A)SN2
B)Nucleophilic addition
C)Electrophilic addition
D)Electrophile elimination
E)Nucleophile elimination

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following can behave as a nucleophile?
A)CH3CH2CH3
B)N(CH3)4+
C)SiH4
D)CH3OH
E)None of the above
A)CH3CH2CH3
B)N(CH3)4+
C)SiH4
D)CH3OH
E)None of the above

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is true about spectator ions?
A)They can be disregarded when drawing mechanisms.
B)They are located in group 4A.
C)They are highly insoluble.
D)They are great targets for nucleophiles.
E)They are highly reactive species.
A)They can be disregarded when drawing mechanisms.
B)They are located in group 4A.
C)They are highly insoluble.
D)They are great targets for nucleophiles.
E)They are highly reactive species.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
The two following molecules can react via a coordination step.Draw the mechanism for this step and the resulting product.Also label the nucleophile and the electrophile. 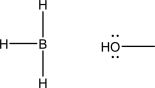


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the carbocation most likely formed after a 1,2-hydride shift? 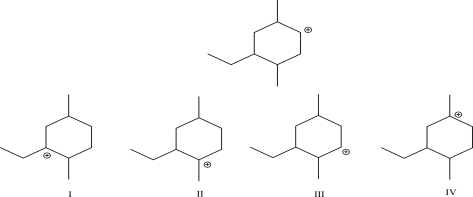
A)I
B)II
C)III
D)IV
E)None of these

A)I
B)II
C)III
D)IV
E)None of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following mechanism steps is most energetically favorable? 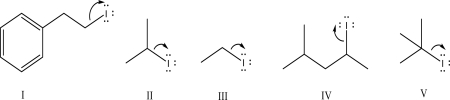
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
For the following reaction,label which reactant is the Lewis base,and supply the missing mechanism arrows. 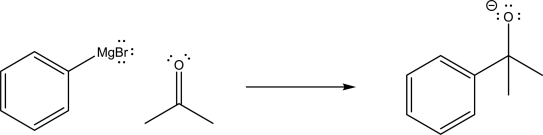


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
The two following species can react via an SN2 mechanism.Fill in the mechanism arrows,and supply the expected products. 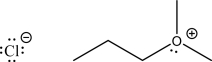


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
The following compounds can react via an E2 step.Draw this mechanism,give the expected products,and label which of the starting materials is the nucleophile and which is the electrophile. 


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules can tautomerize? 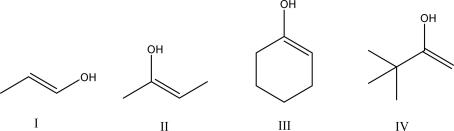
A)I
B)II
C)III
D)IV
E)All of these

A)I
B)II
C)III
D)IV
E)All of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
For the following reaction,label which reactant is the Lewis acid,and supply the missing mechanism arrows. 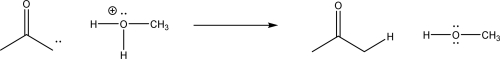


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is an example of electrophile elimination? 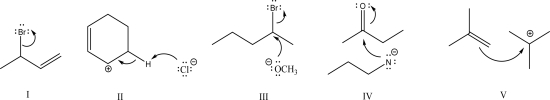
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
The following molecule can undergo heterolysis.Draw the mechanism for this step,and give the most likely products. 


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
The two following molecules can react via a coordination step.Draw the mechanism for this step and the resulting product.Also label the nucleophile and the electrophile. 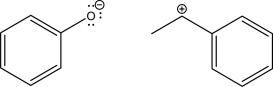


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
In the following reaction,identify all electron-rich and electron-poor sites in the starting materials,and supply the missing mechanism arrows required to form the given product. 


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
In the following reaction,identify all electron-rich and electron-poor sites in the starting materials,and supply the missing mechanism arrows required to form the given product. 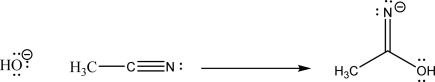
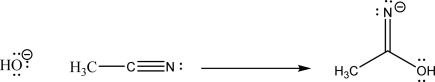

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Add mechanism arrows for the following reactions.Also supply missing electrons in the starting materials and products to complete the mechanism. 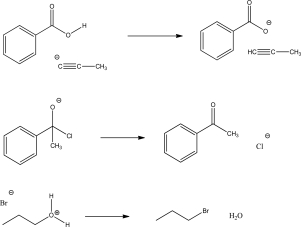


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is an example of electrophilic addition? 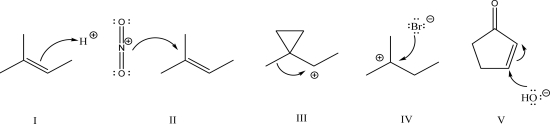
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
The two following species can react via an SN2 mechanism.Fill in the mechanism arrows,and supply the expected products. 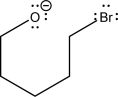


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following 1,2-hydride shift rearrangements is most likely? 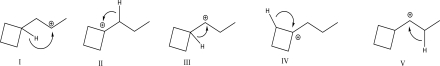
A)I
B)II
C)III
D)IV
E)None of these

A)I
B)II
C)III
D)IV
E)None of these

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a nucleophilic addition step? 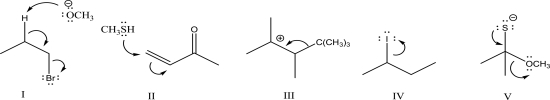
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Explain what is wrong with the following mechanism step,and provide an alternative way in which the two species could react. 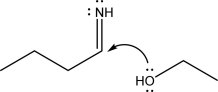


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
Explain what is wrong with the following mechanism step,and provide an alternative way in which the two species could react. 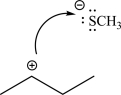


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
There are two possible nucleophile elimination products for the molecule below.Give these products,and draw the nucleophile elimination step that leads to each one. 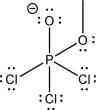


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
The following carbocation undergoes a 1,2-alkyl shift.Draw the mechanism,and give the products of this shift. 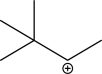


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
The two following species can react via an electrophilic addition step.Draw the mechanism arrows for this step,give the expected product,and label the nucleophile and the electrophile. 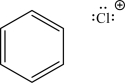


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
The following compounds can react via an E2 step.Draw this mechanism,give the expected products,and label which of the starting materials is the nucleophile and which is the electrophile. 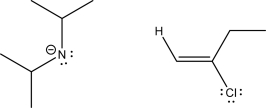


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
The following molecule can undergo heterolysis.Draw the mechanism for this step,and give the most likely products. 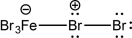
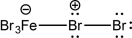

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
Butyl lithium reacts with the following acetyl bromide via a nucleophilic addition step.Provide the omitted mechanism arrows,and draw the missing product in the box. 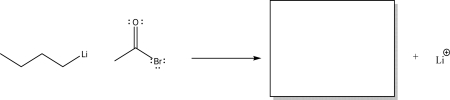


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the keto tautomer for the following compound. 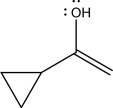


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
For the following reaction scheme,name what type of elementary mechanism step is occurring in Step 1 and Step 2.Choose from the following: SN2,coordination,heterolysis,E2,nucleophilic addition,nucleophile elimination,electrophilic addition,electrophile elimination,carbocation rearrangement,or none of the above. 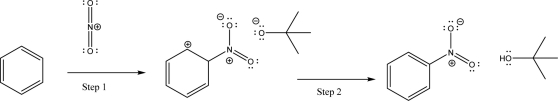


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
The two following species can react via an electrophile elimination step.Draw the mechanism arrows for this step,give the expected product,and label the nucleophile and the electrophile. 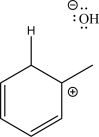


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
For each of the five following mechanism steps,name the step being shown.Choose from the following: proton transfer,SN2,coordination,heterolysis,E2,nucleophilic addition,nucleophile elimination,electrophilic addition,electrophile elimination,carbocation rearrangement,or none of the above. 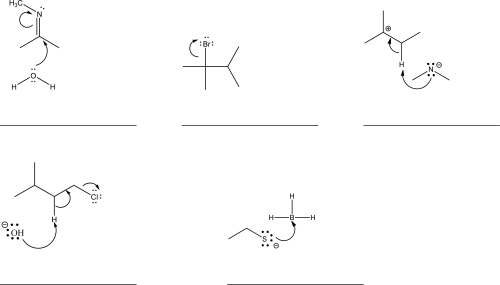


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
The following carbocation undergoes a 1,2-hydride shift.Draw the mechanism,and give the products of this shift. 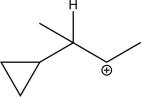


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
Draw the enol tautomer for the following compound. 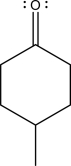


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
The two following species can react via an electrophile elimination step.Draw the mechanism arrows for this step,give the expected product,and label the nucleophile and the electrophile. 


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Suppose that the two following molecules undergo an electrophilic addition step.Draw the mechanism and the product,and label the nucleophile and the electrophile. 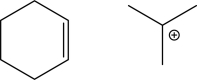


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Explain why the products are favored in the following reaction. 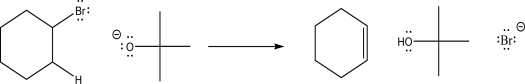


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
The following mechanism proceeds through nucleophile elimination.Fill in the omitted mechanism arrows,and draw the missing product in the box. 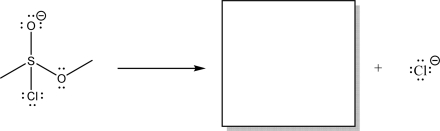


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Indicate which mechanism step is shown below,and draw the expected product(s). 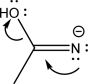


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
The two following molecules can react via a nucleophilic addition.Draw the mechanism arrows for this step,and give the products. 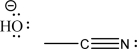
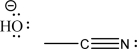

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


