Deck 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 10: An Introduction to Multistep Mechanisms: Sn1 and E1 Reactions
1
What is the rate law for the following SN2 reaction? ![<strong>What is the rate law for the following S<sub>N</sub>2 reaction? </strong> A)Rate = k[A] B)Rate = k[B] C)Rate = k[A]<sup>2</sup> D)Rate = k[B]<sup>2</sup> E)Rate = k[A][B]](https://storage.examlex.com/TB4360/11ea8a0a_379b_ba89_9fff_3f750a61c965_TB4360_00.jpg)
A)Rate = k[A]
B)Rate = k[B]
C)Rate = k[A]2
D)Rate = k[B]2
E)Rate = k[A][B]
![<strong>What is the rate law for the following S<sub>N</sub>2 reaction? </strong> A)Rate = k[A] B)Rate = k[B] C)Rate = k[A]<sup>2</sup> D)Rate = k[B]<sup>2</sup> E)Rate = k[A][B]](https://storage.examlex.com/TB4360/11ea8a0a_379b_ba89_9fff_3f750a61c965_TB4360_00.jpg)
A)Rate = k[A]
B)Rate = k[B]
C)Rate = k[A]2
D)Rate = k[B]2
E)Rate = k[A][B]
Rate = k[A][B]
2
Which of the following is true regarding SN1 reactions?
A)The stereochemistry of SN1 products has an R configuration exclusively.
B)The stereochemistry of SN1 products has an S configuration exclusively.
C)SN1 reactions produce racemic products in achiral environments.
D)SN1 reactions are stereospecific.
E)SN1 reactions produce cis isomers exclusively.
A)The stereochemistry of SN1 products has an R configuration exclusively.
B)The stereochemistry of SN1 products has an S configuration exclusively.
C)SN1 reactions produce racemic products in achiral environments.
D)SN1 reactions are stereospecific.
E)SN1 reactions produce cis isomers exclusively.
SN1 reactions produce racemic products in achiral environments.
3
What is the product of the following E2 reaction? 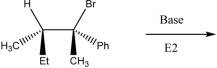
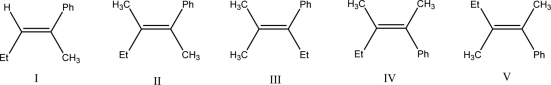
A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V
IV
4
Which of the following steps could be found in an E1 mechanism? 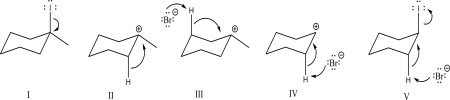
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following could be the rate law for an SN1 reaction?
A)Rate = k[A]
B)Rate = k[A]2
C)Rate = k[A][B]
D)Rate = k[A][B]2
E)Rate = k[A]2[B]2
A)Rate = k[A]
B)Rate = k[A]2
C)Rate = k[A][B]
D)Rate = k[A][B]2
E)Rate = k[A]2[B]2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following steps could be found in an E1 mechanism? 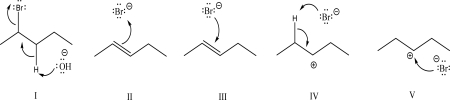
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
What is the most likely product of the following SN2 reaction? 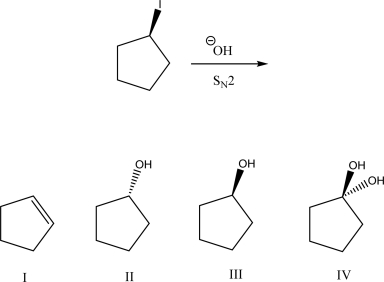
A)I
B)II
C)III
D)IV
E)A racemic mixture of II and III

A)I
B)II
C)III
D)IV
E)A racemic mixture of II and III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
The following compound can undergo two successive SN2 reactions.What are the products of these reactions? 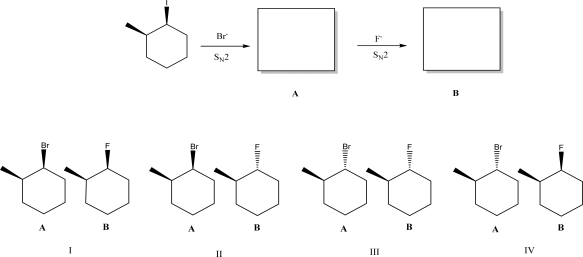
A)I
B)II
C)III
D)IV
E)None of these pairs

A)I
B)II
C)III
D)IV
E)None of these pairs

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Which is not true of a free energy diagram for an SN1 reaction?
A)It includes local energy minima between transition states.
B)It includes intermediates that are higher in energy than reactants or products.
C)It includes two transition states.
D)The heteolysis step always has the highest energy barrier.
E)All of the above are true.
A)It includes local energy minima between transition states.
B)It includes intermediates that are higher in energy than reactants or products.
C)It includes two transition states.
D)The heteolysis step always has the highest energy barrier.
E)All of the above are true.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
What is the most likely product of the following SN1 reaction? 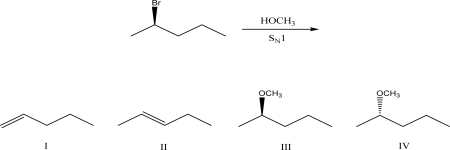
A)I
B)II
C)III
D)IV
E)A mixture of III and IV

A)I
B)II
C)III
D)IV
E)A mixture of III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which is true of a free energy diagram for an E1 reaction?
A)It proceeds through a carbocation intermediate.
B)It begins with the rate-determining step.
C)It includes two transition states.
D)It ends with alkene-containing products.
E)All of the above are true.
A)It proceeds through a carbocation intermediate.
B)It begins with the rate-determining step.
C)It includes two transition states.
D)It ends with alkene-containing products.
E)All of the above are true.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which is true of a free energy diagram for an SN1 reaction?
A)It shows one transition state.
B)It always shows that the reaction is net exothermic.
C)It never shows intermediates.
D)It always shows that the reaction is net endothermic.
E)It always includes multiple energy barriers.
A)It shows one transition state.
B)It always shows that the reaction is net exothermic.
C)It never shows intermediates.
D)It always shows that the reaction is net endothermic.
E)It always includes multiple energy barriers.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
For the rate law given below,what would happen to the rate of a reaction if the concentrations of [A] and [B] were doubled?
Rate = k[A][B]
A)The rate would stay the same.
B)The rate would double.
C)The rate would triple.
D)The rate would quadruple.
E)Not enough information is given to know.
Rate = k[A][B]
A)The rate would stay the same.
B)The rate would double.
C)The rate would triple.
D)The rate would quadruple.
E)Not enough information is given to know.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following steps could be found in an SN1 mechanism? 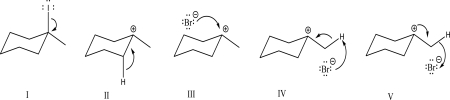
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
What is the product of the following E2 reaction? 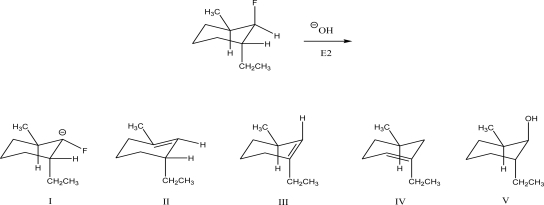
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which is the rate law for the following E1 reaction? ![<strong>Which is the rate law for the following E1 reaction? </strong> A)Rate = k[A] B)Rate = k[B] C)Rate = k[A]<sup>2</sup> D)Rate = k[B]<sup>2</sup> E)Rate = k[A][B]](https://storage.examlex.com/TB4360/11ea8a0a_379b_4557_9fff_afb62a37d8b3_TB4360_00.jpg)
A)Rate = k[A]
B)Rate = k[B]
C)Rate = k[A]2
D)Rate = k[B]2
E)Rate = k[A][B]
![<strong>Which is the rate law for the following E1 reaction? </strong> A)Rate = k[A] B)Rate = k[B] C)Rate = k[A]<sup>2</sup> D)Rate = k[B]<sup>2</sup> E)Rate = k[A][B]](https://storage.examlex.com/TB4360/11ea8a0a_379b_4557_9fff_afb62a37d8b3_TB4360_00.jpg)
A)Rate = k[A]
B)Rate = k[B]
C)Rate = k[A]2
D)Rate = k[B]2
E)Rate = k[A][B]

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following steps could be found in an SN1 mechanism? 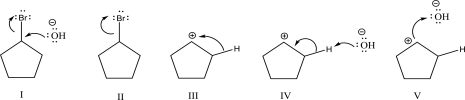
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
For the rate law given below,what would happen to the rate of a reaction if the concentration of [A] was doubled?
Rate = k[A][B]
A)The rate would slow by a factor of ½.
B)The rate would stay the same.
C)The rate would double.
D)The rate would triple.
E)The rate would quadruple.
Rate = k[A][B]
A)The rate would slow by a factor of ½.
B)The rate would stay the same.
C)The rate would double.
D)The rate would triple.
E)The rate would quadruple.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
The following rate law could be possible for which of the following mechanisms? 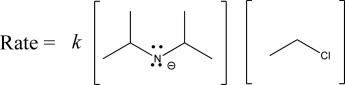
A)E1
B)E2
C)SN1
D)SN2
E)E2 and SN2

A)E1
B)E2
C)SN1
D)SN2
E)E2 and SN2

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is true of SN1 reactions?
A)They are stereospecific.
B)They occur in a single step.
C)They are second order.
D)They proceed through a carbocation intermediate.
E)Their rate is dependent on the concentration of attacking nucleophile.
A)They are stereospecific.
B)They occur in a single step.
C)They are second order.
D)They proceed through a carbocation intermediate.
E)Their rate is dependent on the concentration of attacking nucleophile.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Draw the product(s)of the following intramolecular SN1 reaction. 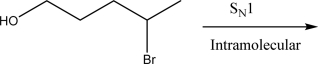


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
The following alkyl bromide can react with NaF via an SN1 mechanism.Draw the mechanism and product(s)of this reaction.Be sure to pay attention to stereochemistry,if applicable. 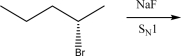


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following will not undergo a 1,2-hydride shift? 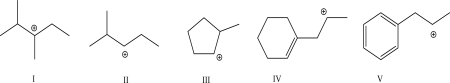
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following proton transfers is most likely to occur in an acidic solution? 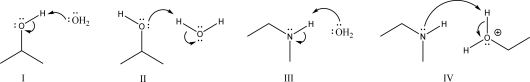
A)I
B)II
C)III
D)IV
E)All of these are likely in an acidic solution.

A)I
B)II
C)III
D)IV
E)All of these are likely in an acidic solution.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Draw a detailed free energy diagram for the following SN1 reaction.Include and label the overall reactants,the overall products,the intermediate(s),the axis,and the transition state(s). 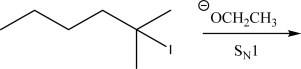


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following mechanism steps is not allowed? 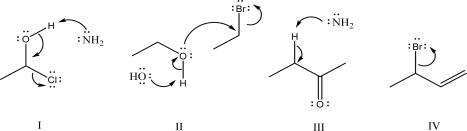
A)I
B)II
C)III
D)IV
E)All are allowed.

A)I
B)II
C)III
D)IV
E)All are allowed.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would proceed though a carbocation rearrangement during an E1 reaction? 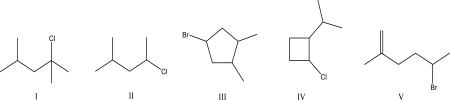
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
Draw a detailed free energy diagram for the following SN1 reaction.Include and label the overall reactants,the overall products,the intermediate(s),the axis,and the transition state(s). 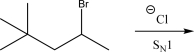


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Draw a detailed free energy diagram for the following E1 reaction.Include and label the overall reactants,the overall products,the intermediate(s),the axis,and the transition state(s). 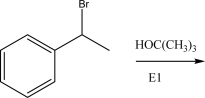


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the following E1 reaction? 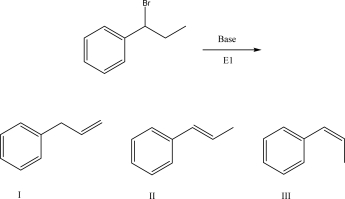
A)I
B)II
C)III
D)A mixture of II and III
E)An E1 reaction mechanism is not possible.

A)I
B)II
C)III
D)A mixture of II and III
E)An E1 reaction mechanism is not possible.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
The following alkyl halide can react with NaBr via an SN1 mechanism.Draw the product(s)of this reaction.Be sure to pay attention to stereochemistry,if applicable. 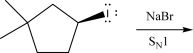


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Draw the product(s)of the following E2 reaction. 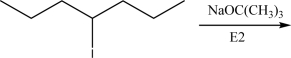


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following would not proceed though a carbocation rearrangement during an SN1 reaction? 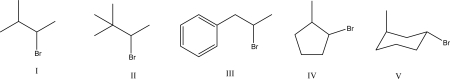
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
If the following compound undergoes a 1,2-hydride shift,what is the most likely product? 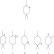
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
For the following E2 reaction,what would happen to the rate if the concentration of A was doubled? 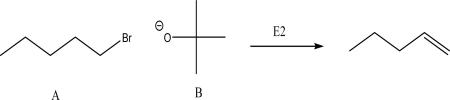


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is true of intramolecular proton transfers?
A)They can occur only in basic conditions.
B)They can occur only in acidic conditions.
C)They can occur in both acidic and basic conditions.
D)They are the most common way in which protons are transferred.
E)They are not likely to occur.
A)They can occur only in basic conditions.
B)They can occur only in acidic conditions.
C)They can occur in both acidic and basic conditions.
D)They are the most common way in which protons are transferred.
E)They are not likely to occur.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would undergo a 1,2-methyl shift? 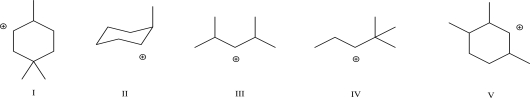
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Give the rate law and order for the following E1 reaction. 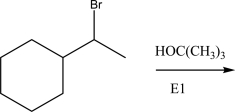


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
With regard to rate laws,provide the order for E1,E2,SN1,and SN2 reactions.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
Draw the mechanism and the product(s)for the following E1 reaction. 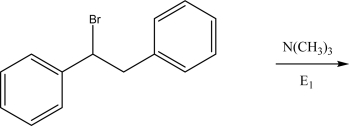


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
For the following SN2 reaction,what would happen to the rate if the concentrations of both A and B were tripled? 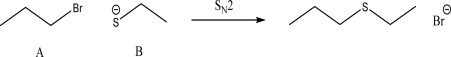


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the product(s)of the following E1 reaction. 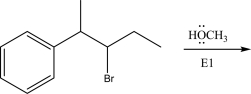


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
The following mechanism step is not reasonable.Explain why. 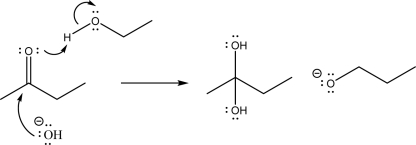


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
When a leaving group and a hydrogen atom are 180° apart,they are considered to be _______.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
The following mechanism has an unreasonable step.Indicate which step is unreasonable,and explain why. 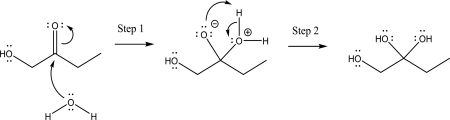


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the product(s)of the following SN1 reaction. 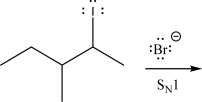


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following reactions produces a new stereocenter with unequal amounts of R and S configurations? Explain. 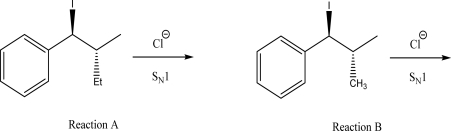


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
SN2 reactions require ________ attack of the substrate by the nucleophile.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
Explain why the following carbocation rearrangement is not likely. 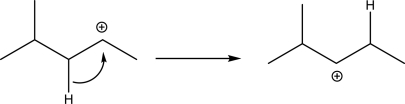


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
Draw the product(s)of the following E2 reaction. 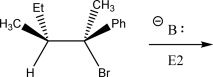


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
Are mechanism steps requiring three species likely? Explain why or why not.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
The following mechanism has an unreasonable step.Indicate which step is unreasonable,and explain why. 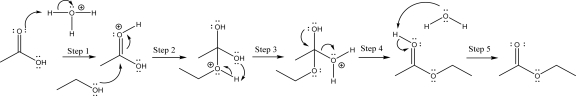


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
A new tetrahedral stereocenter is produced in a step in which the reactants and the environment are achiral.How much of the R configuration can be expected?

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
A reaction that produces a new stereocenter with only a single configuration is called _______.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
Explain why the following carbocation rearrangement is not likely. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
For the following SN1 reaction,what would happen to the rate if the concentration of B was quadrupled? 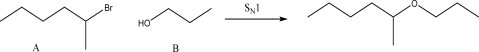


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the product(s)of the following SN1 reaction. 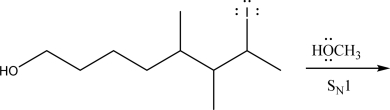


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
Draw the product(s)of the following E1 reaction. 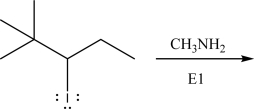


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the product(s)of the following E2 reaction. 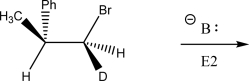


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


