Deck 15: Organic Synthesis 1: Beginning Concepts
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 15: Organic Synthesis 1: Beginning Concepts
1
What is the missing starting material in the following synthetic sequence? 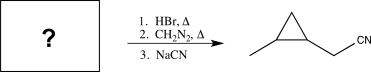
A)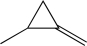
B)
C)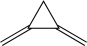
D)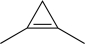
E)

A)

B)

C)

D)

E)


2
Which of the following steps would result in an alteration of the carbon skeleton?
A)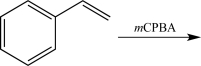
B)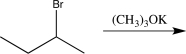
C)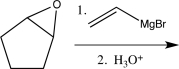
D)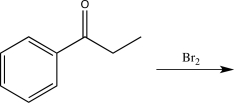
E)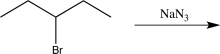
A)

B)

C)

D)

E)


3
Fill in the missing precursor to complete the transform below. 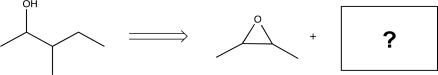
A)CH3Br
B)CH3MgBr
C)(CH3)3MgBr
D)CH3CH2Br
E)CH3CH2MgBr

A)CH3Br
B)CH3MgBr
C)(CH3)3MgBr
D)CH3CH2Br
E)CH3CH2MgBr
CH3CH2MgBr
4
Determine the major product of the following reaction sequence. 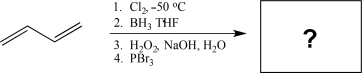
A)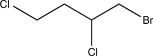
B)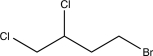
C)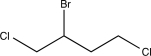
D)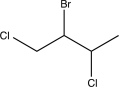
E)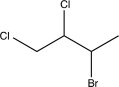

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following solvents would not be a good choice to use when carrying out the reaction below? 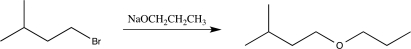
A)DMSO
B)CH3CH2CH2OH
C)CH3OH
D)DMF
E)Ethyl acetate

A)DMSO
B)CH3CH2CH2OH
C)CH3OH
D)DMF
E)Ethyl acetate

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the final product of the reaction sequence shown below. 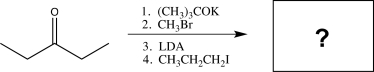
A)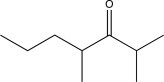
B)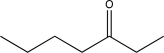
C)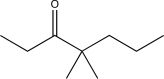
D)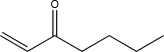
E)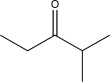
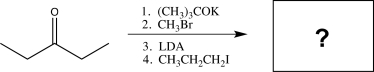
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following should not be included in a synthetic scheme?
A)Mechanism details
B)Reagents
C)Solvent
D)Reaction temperature
E)Reaction time
A)Mechanism details
B)Reagents
C)Solvent
D)Reaction temperature
E)Reaction time

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following synthetic steps is written correctly and would lead to the product shown?
A)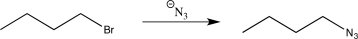
B)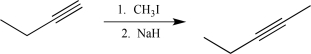
C)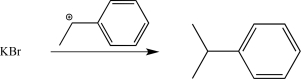
D)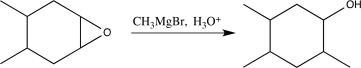
E)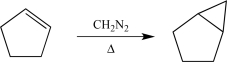
A)

B)

C)

D)
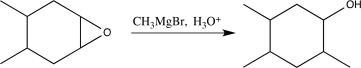
E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Choose the best precursors that could be used to synthesize the target molecule in the forward direction. 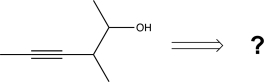
A)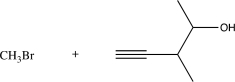
B)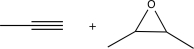
C)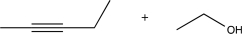
D)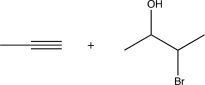
E)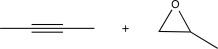

A)
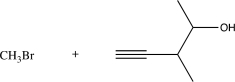
B)
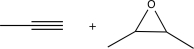
C)
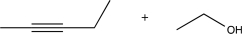
D)
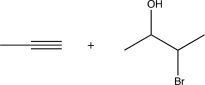
E)
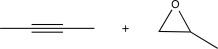

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is appropriate to include in a synthetic step but should not be included in the mechanism for a reaction?
A)Starting materials
B)Mechanism arrows
C)Products
D)Reaction temperature
E)Necessary reagents
A)Starting materials
B)Mechanism arrows
C)Products
D)Reaction temperature
E)Necessary reagents

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Determine the missing precursor needed to synthesize the molecule below. 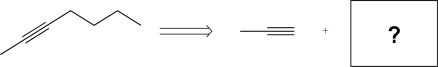
A)
B)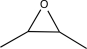
C)
D)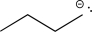
E)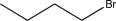

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following would not alter the carbon skeleton of the starting material?
A)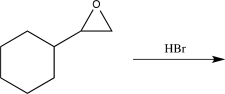
B)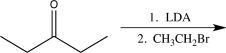
C)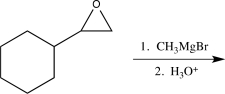
D)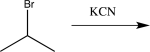
E)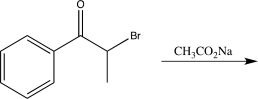
A)

B)
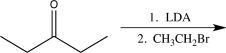
C)
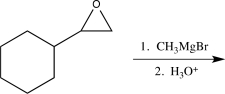
D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following synthetic steps shows a reagent that is written incorrectly?
A)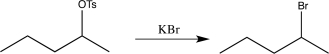
B)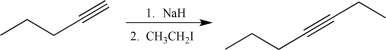
C)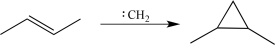
D)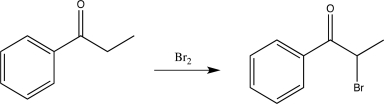
E)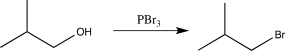
A)

B)

C)
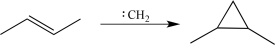
D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following synthetic steps would not be classified as a functional group conversion?
A)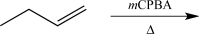
B)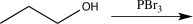
C)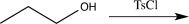
D)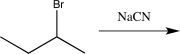
E)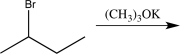
A)
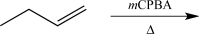
B)
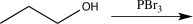
C)
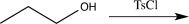
D)

E)
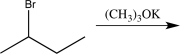

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following would not be a reasonable precursor of the target shown below? 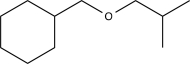
A)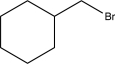
B)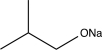
C)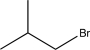
D)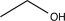
E)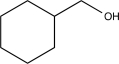

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following transforms would lead to a synthetic trap when carried out in the forward direction?
A)
B)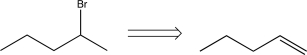
C)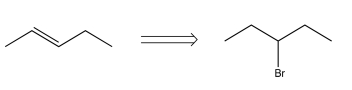
D)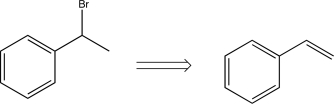
E)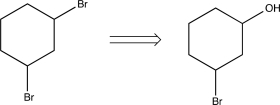
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following transforms would not be a viable synthesis in the forward direction?
A)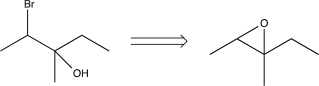
B)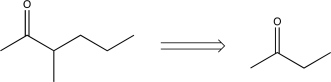
C)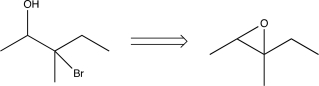
D)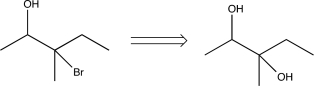
E)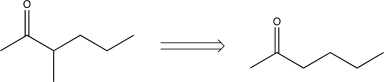
A)

B)
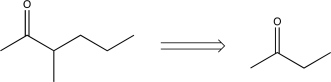
C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Determine the missing starting material for the following synthetic step. 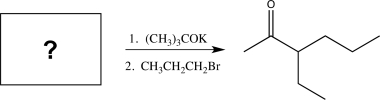
A)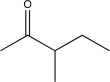
B)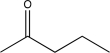
C)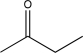
D)
E)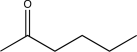

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the best reaction or sequence of reactions to carry out the following synthetic transformation. 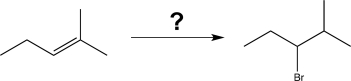
A)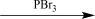
B)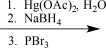
C)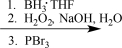
D)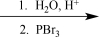
E)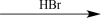

A)
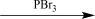
B)
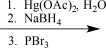
C)
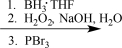
D)
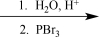
E)
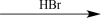

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following solvents would be the best choice to use when carrying out the reaction below? 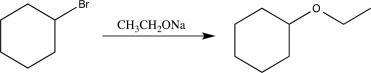
A)H2O
B)CH3OH
C)Acetic acid
D)CH3CH2OH
E)Cyclohexanol

A)H2O
B)CH3OH
C)Acetic acid
D)CH3CH2OH
E)Cyclohexanol

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Show how the following could be synthesized from two separate alkynes,each with no more than four carbon atoms. 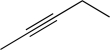


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Determine the necessary starting material to complete the step below. 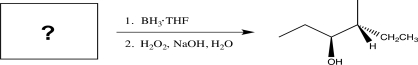
A)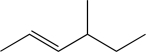
B)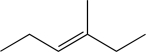
C)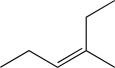
D)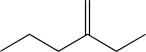
E)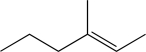

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Fill in the missing starting material needed to complete the following synthetic step. 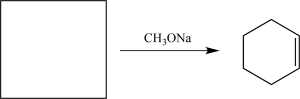


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Determine the missing starting material that would exclusively yield the product shown below. 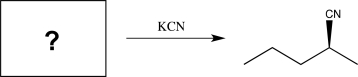
A)
B)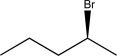
C)
D)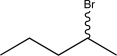
E)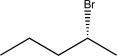

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Show how to carry out the following synthesis. 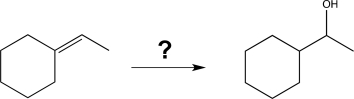


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Briefly explain why the synthetic step shown below is incorrect,and rewrite it so that it is correct. 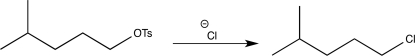


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
How do the overall percent yields of the linear and convergent syntheses shown below compare? 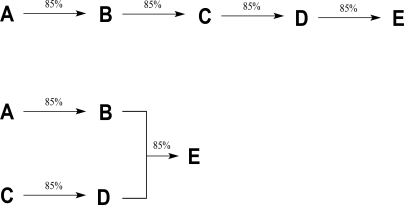
A)The convergent synthesis has a higher overall percent yield than the linear synthesis.
B)The linear synthesis has a higher overall percent yield than the convergent synthesis.
C)Both synthetic schemes result in the same overall percent yield.
D)It is not possible to determine how yields compare based on the information given.
E)It depends on how many carbons are in each compound.

A)The convergent synthesis has a higher overall percent yield than the linear synthesis.
B)The linear synthesis has a higher overall percent yield than the convergent synthesis.
C)Both synthetic schemes result in the same overall percent yield.
D)It is not possible to determine how yields compare based on the information given.
E)It depends on how many carbons are in each compound.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
A researcher synthesizes 2-bromo-2-phenylpentane by reacting 2-phenyl-2-tosylpentane with potassium bromide.Write a synthetic step that shows this reaction,and draw the mechanism.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Supply the missing reagents necessary to complete the synthetic steps shown below,and indicate whether each step is a functional group conversion or a carbon-carbon bond formation reaction. 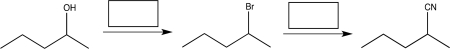


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Determine the overall percent yield for a five-step synthesis in which the yield of each individual step is 90%.
A)90%
B)45%
C)59%
D)81%
E)33%
A)90%
B)45%
C)59%
D)81%
E)33%

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Show how the following could be synthesized from two different alkyl bromides. 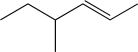


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Supply the missing reagents necessary to complete the following sequence. 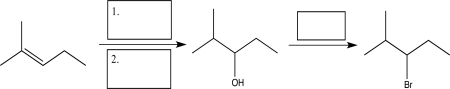


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Fill in the missing reagent to complete the following synthetic step. 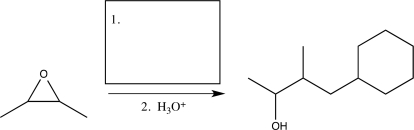


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
A student proposes the following synthesis.Explain why this reaction would not work as intended. 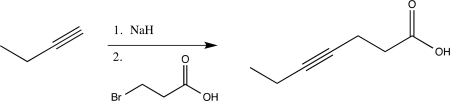


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Propose a way to carry out the synthetic transformation shown below (may require more than one step). 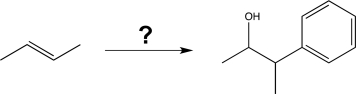


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Write a synthetic step that shows the following: A researcher subjects 2-bromoheptane to potassium tert-butoxide with tert-butanol as the solvent.The major product of the reaction is 1-heptene.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would yield the compound shown via an E2 reaction? 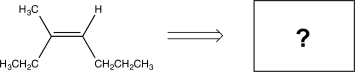
A)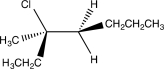
B)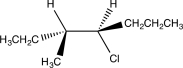
C)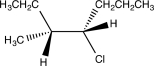
D)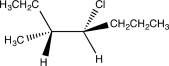
E)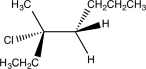

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Draw two possible sets of precursors that could be used to synthesize the target molecule below. 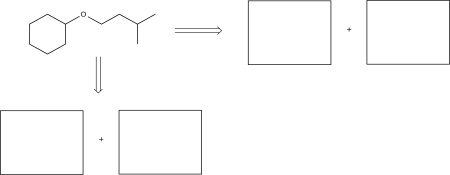


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Write a synthetic step that shows the following reaction: 3-pentanone was treated first with lithium diisopropylamide.After this reaction was allowed to reach completion,the resulting product was treated with ethyl bromide to yield 4-methyl-3-hexanone as the overall product.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Determine the alkyl bromide and alkyne that could be used to synthesize the target molecule below. 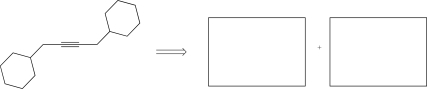


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Determine an appropriate synthetic step that could be used to carry out the transform below in the forward direction. 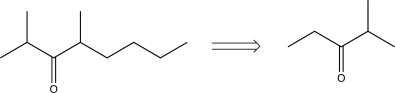


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the overall percent yield of the following synthetic sequence. 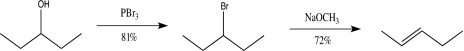


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Show how the following target could be synthesized using only compounds containing three or fewer carbon atoms as the sources of carbon. 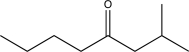


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Two students need to carry out the synthetic step shown below.One plans to use ethanol as the solvent,while the other opts for methanol.Which is the better choice of solvent,and why? 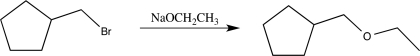


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Show how the following transform could be carried out in the forward direction. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Show how the following synthetic transformation could be carried out. 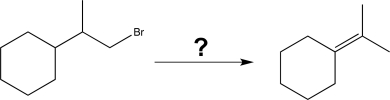


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Show how you would carry out the following transformation. 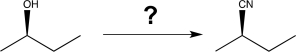
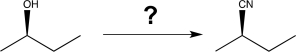

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Design two different synthetic routes to produce the target compound shown.One route should start with epoxide and the other should start from a diene. 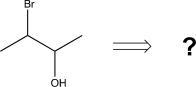


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Fill in the missing reagents and intermediates in the synthetic sequence below. 


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
A student proposes the following synthetic step.Explain the potential error in this proposed step,and suggest a better alternative to carry out the desired synthetic transformation. 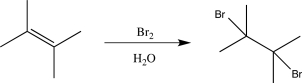


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


