Deck 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/54
Play
Full screen (f)
Deck 16: Orbital Interactions 2: Extended Π Systems,conjugation,and Aromaticity
1
Which one of the following compounds is aromatic? 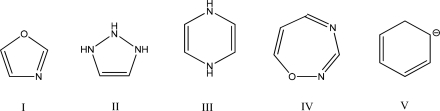
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
I
2
Which atomic orbital contributor has three nodes? 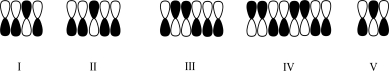
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
IV
3
Which term can be used to define a molecule that is cyclic,planar,completely conjugated,and has four π electrons.
A)Aromatic
B)Antiaromatic
C)Nonaromatic
D)Semiaromatic
E)None of the above
A)Aromatic
B)Antiaromatic
C)Nonaromatic
D)Semiaromatic
E)None of the above
Antiaromatic
4
Which of the following reactions releases the least amount of heat upon hydrogenation? 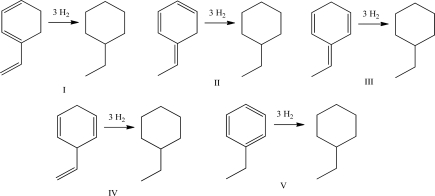
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
5
How many of the following compounds are aromatic? 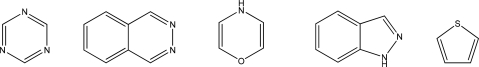
A)Zero
B)One
C)Two
D)Three
E)Four

A)Zero
B)One
C)Two
D)Three
E)Four

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
6
Assuming all of the following molecules are planar,which one can be labeled antiaromatic? 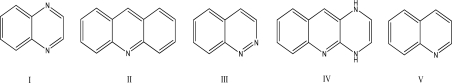
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
7
Which molecular orbital has the lowest energy?
A)The highest bonding MO
B)The highest antibonding MO
C)The lowest bonding MO
D)HOMO
E)LUMO
A)The highest bonding MO
B)The highest antibonding MO
C)The lowest bonding MO
D)HOMO
E)LUMO

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
8
Assume that a compound is a cyclic,planar,completely conjugated ring.Which number of π electrons would make it aromatic?
A)0 π electrons
B)2 π electrons
C)3 π electrons
D)4 π electrons
E)32 π electrons
A)0 π electrons
B)2 π electrons
C)3 π electrons
D)4 π electrons
E)32 π electrons

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
9
Which atomic orbital contributor has three areas of constructive interference? 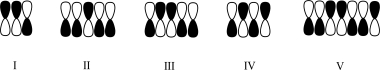
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
10
Which hydrogen has the lowest Pka? 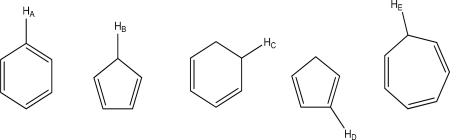
A)HA
B)HB
C)HC
D)HD
E)HE

A)HA
B)HB
C)HC
D)HD
E)HE

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following molecules is aromatic? 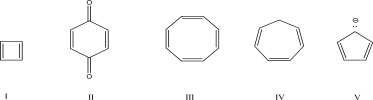
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
12
Which number of π electrons can be defined as an anti-Hückel number?
A)0
B)1
C)2
D)3
E)22
A)0
B)1
C)2
D)3
E)22

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following molecules is aromatic? 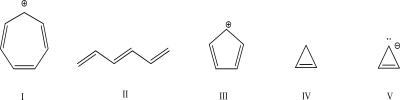
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
14
Which atomic orbital contributor corresponds to the HOMO of a molecule with four π electrons in a conjugated system? 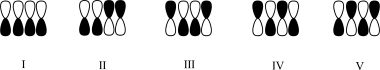
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
15
Which atomic orbital contributor has the highest energy? 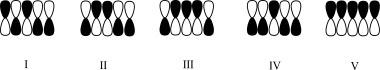
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
16
How many areas of constructive overlap are in the following atomic orbital contributor? 
A)Zero
B)One
C)Two
D)Three
E)Four

A)Zero
B)One
C)Two
D)Three
E)Four

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
17
Which atomic orbital contributor has the lowest energy? 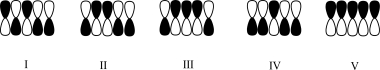
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
18
Assuming all of the following molecules are planar,which one can be labeled antiaromatic? 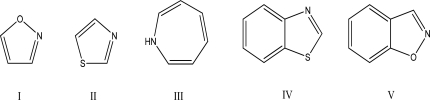
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following reactions releases the least amount of heat upon hydrogenation? 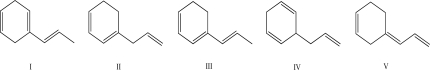
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following molecules is aromatic? 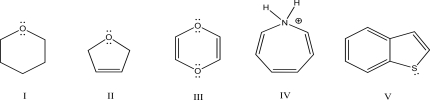
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
21
How many electrons are in the following molecule's largest conjugated π system? 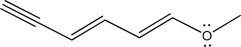
A)Two
B)Four
C)Six
D)Eight
E)Ten

A)Two
B)Four
C)Six
D)Eight
E)Ten

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
22
Rank the three following molecules by the amount of heat released upon hydrogenation (from lowest amount to highest amount).Explain your answer. 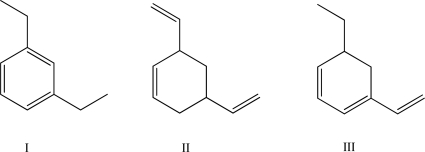


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
23
Which hydrogen has the lowest Pka? 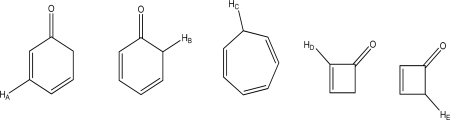
A)HA
B)HB
C)HC
D)HD
E)HE

A)HA
B)HB
C)HC
D)HD
E)HE

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
24
Rank the three following molecules by the amount of heat released upon hydrogenation (from lowest amount to highest amount).Explain your answer. 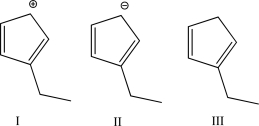


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
25
Use the Frost method to explain whether furan,diagramed below is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 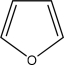


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
26
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 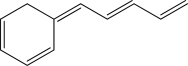


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
27
Give the four requirements a molecule must have to be considered aromatic.

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the areas of constructive interference for the following atomic contributor. 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
29
Which heterolysis step will occur slowest? 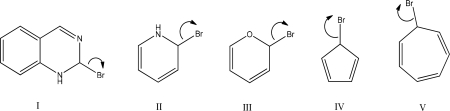
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
30
How many nodes does the following atomic contributor have? 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the areas of constructive interference for the following atomic contributor. 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
32
Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 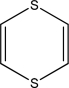


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
33
How many nodes does the following atomic contributor have? 


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
34
How many electrons are in the given molecule's largest conjugated π system? 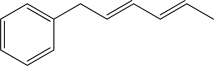
A)Four
B)Five
C)Six
D)Eight
E)Ten

A)Four
B)Five
C)Six
D)Eight
E)Ten

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
35
For the following molecule,give the number of π electrons and nonbonding electrons. 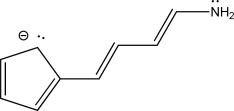


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
36
How many electrons are in the following molecule's largest conjugated π system? 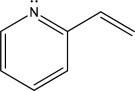
A)Two
B)Four
C)Six
D)Eight
E)Ten

A)Two
B)Four
C)Six
D)Eight
E)Ten

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
37
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 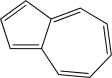


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
38
How many electrons are in the following molecule's largest conjugated π system? 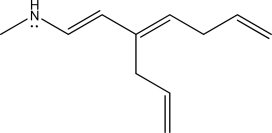
A)Two
B)Four
C)Six
D)Eight
E)Ten

A)Two
B)Four
C)Six
D)Eight
E)Ten

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
39
Which heterolysis step will occur fastest? 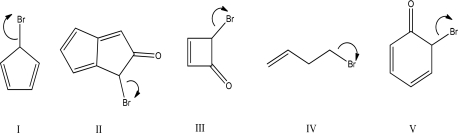
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
40
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 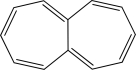


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
41
Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 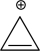


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
42
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 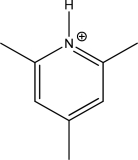


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
43
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 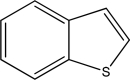


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
44
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 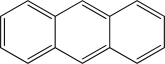


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
45
How many different π systems does the following molecule contain? 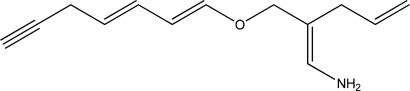


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
46
For the following molecule,give the number of π electrons and the number of nonbonding electrons. 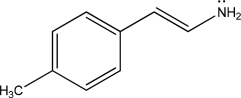


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
47
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 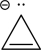
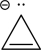

Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
48
Which is more acidic,HA or HB? Explain. 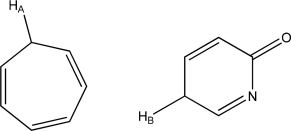


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
49
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 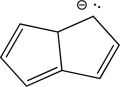


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
50
Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 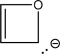


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
51
Which is more acidic,HA or HB? Explain. 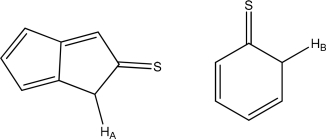


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
52
Is the following compound aromatic,antiaromatic,or nonaromatic? Explain your answer. 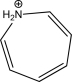


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
53
How many different π systems does the following molecule contain? Give the number of electrons in each. 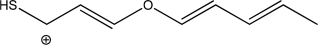


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck
54
Assume that the following molecule is planar.Use the Frost method to explain whether it is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 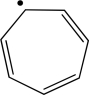


Unlock Deck
Unlock for access to all 54 flashcards in this deck.
Unlock Deck
k this deck


