Deck 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 25: Electrophilic Aromatic Substitution 2: Substitution on Mono- and Disubstituted Benzene and Other Aromatic Rings
1
Which of the following would undergo nitration at the highest rate?
A)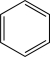
B)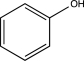
C)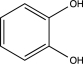
D)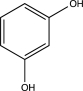
E)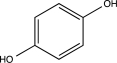
A)

B)

C)

D)

E)


2
Which of the following resonance structures is least stable?
A)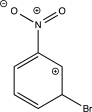
B)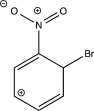
C)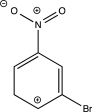
D)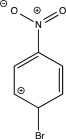
E)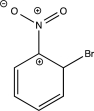
A)

B)

C)

D)

E)


3
Which of the following is an intermediate in the mechanism for the reaction below? 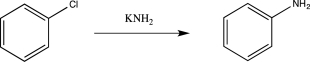
A)
B)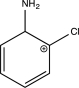
C)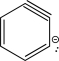
D)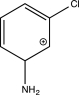
E)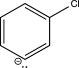

A)

B)

C)

D)

E)


4
Which of the following statements is true regarding the two reactions shown below? 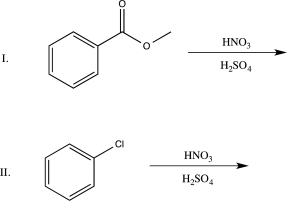
A)Both reactions will occur at a slower rate than the nitration of benzene, and both will yield the meta-substituted product.
B)Both reactions will occur at a faster rate than the nitration of benzene. Reaction I will yield ortho/para-substituted products, and Reaction II will yield the meta-substituted product.
C)Both reactions will yield meta-substituted products. Reaction I will be slower than the nitration of benzene, while Reaction II will be faster.
D)Both reactions will yield ortho/para-substituted products. Reaction I will be faster than the nitration of benzene, while Reaction II will be slower.
E)Both reactions will occur at a slower rate than the nitration of benzene. Reaction I will yield the meta-substituted product, and Reaction II will yield ortho/para-substituted products.

A)Both reactions will occur at a slower rate than the nitration of benzene, and both will yield the meta-substituted product.
B)Both reactions will occur at a faster rate than the nitration of benzene. Reaction I will yield ortho/para-substituted products, and Reaction II will yield the meta-substituted product.
C)Both reactions will yield meta-substituted products. Reaction I will be slower than the nitration of benzene, while Reaction II will be faster.
D)Both reactions will yield ortho/para-substituted products. Reaction I will be faster than the nitration of benzene, while Reaction II will be slower.
E)Both reactions will occur at a slower rate than the nitration of benzene. Reaction I will yield the meta-substituted product, and Reaction II will yield ortho/para-substituted products.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which sequence of reactions would be the best choice to carry out the step below? 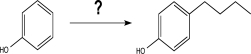
A)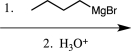
B)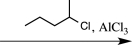
C)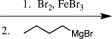
D)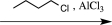
E)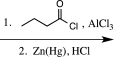

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following would produce a mixture of two isomers as the major products when allowed to react with Cl2 and AlCl3?
A)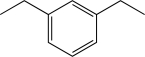
B)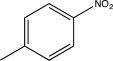
C)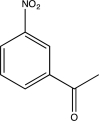
D)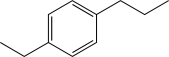
E)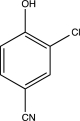
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following substituents is an activating group?
A)
B)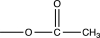
C)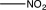
D)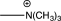
E)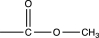
A)

B)
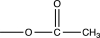
C)
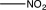
D)
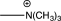
E)
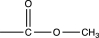

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following substituents is an ortho/para-directing group?
A)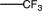
B)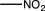
C)
D)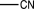
E)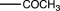
A)
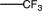
B)
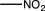
C)

D)
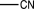
E)
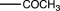

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
How many of the following molecules will undergo chlorination at a higher rate than benzene and will produce ortho/para-substituted products? 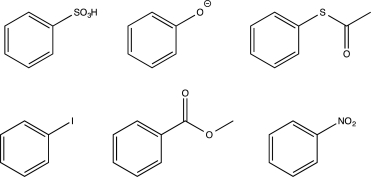
A)One
B)Two
C)Three
D)Four
E)Five

A)One
B)Two
C)Three
D)Four
E)Five

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Which structure represents a major intermediate in the chlorination of nitrobenzene?
A)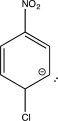
B)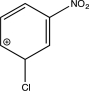
C)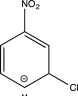
D)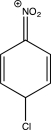
E)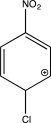
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following correctly explains why alkyl groups are ortho/para-directing groups?
A)Meta substitution results in an arenium ion in which a positive charge is destabilized by an adjacent electron-withdrawing group.
B)Ortho/para substitution results in an arenium ion intermediate in which all atoms have complete octets.
C)A lone pair of electrons on the alkyl group participates in resonance and stabilizes the arenium ion intermediate in ortho/para substitution.
D)The most stable resonance structure for the arenium ion intermediate in ortho/para substitution contains a 2° carbocation.
E)The alkyl group is electron donating via induction and stabilizes the adjacent positive charge in the ortho/para substitution intermediate.
A)Meta substitution results in an arenium ion in which a positive charge is destabilized by an adjacent electron-withdrawing group.
B)Ortho/para substitution results in an arenium ion intermediate in which all atoms have complete octets.
C)A lone pair of electrons on the alkyl group participates in resonance and stabilizes the arenium ion intermediate in ortho/para substitution.
D)The most stable resonance structure for the arenium ion intermediate in ortho/para substitution contains a 2° carbocation.
E)The alkyl group is electron donating via induction and stabilizes the adjacent positive charge in the ortho/para substitution intermediate.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following substituents is an activating ortho/para-directing group?
A)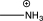
B)
C)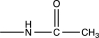
D)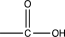
E)
A)

B)

C)
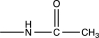
D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
How many isomeric products could result from the reaction below? 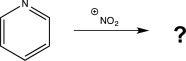
A)One
B)Two
C)Three
D)Four
E)Five

A)One
B)Two
C)Three
D)Four
E)Five

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Predict the major product of the following reaction. 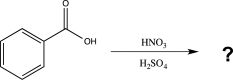
A)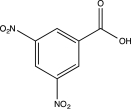
B)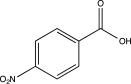
C)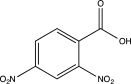
D)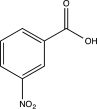
E)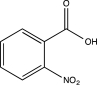

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
In which of the following arenium ions do all atoms have a complete octet?
A)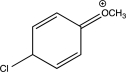
B)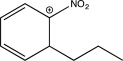
C)
D)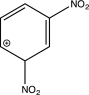
E)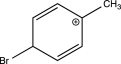
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the correct order of increasing rate of bromination for the three molecules below? 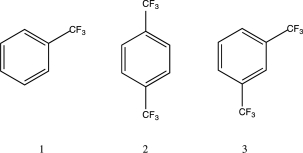
A)2 < 3 < 1
B)1 < 2 < 3
C)2 < 1 < 3
D)3 < 2 < 1
E)3 < 1 < 2

A)2 < 3 < 1
B)1 < 2 < 3
C)2 < 1 < 3
D)3 < 2 < 1
E)3 < 1 < 2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Predict the major product of the following reaction. 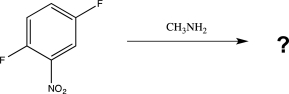
A)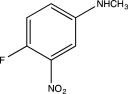
B)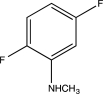
C)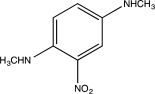
D)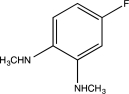
E)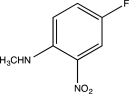

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following arenium ions is most stable?
A)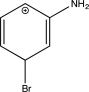
B)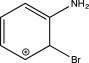
C)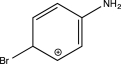
D)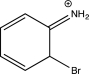
E)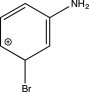
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the following from slowest to fastest rate of nitration. 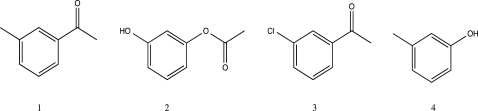
A)1 < 3 < 2 < 4
B)3 < 1 < 2 < 4
C)3 < 1 < 4 < 2
D)2 < 4 < 1 < 3
E)4 < 3 < 2 < 1

A)1 < 3 < 2 < 4
B)3 < 1 < 2 < 4
C)3 < 1 < 4 < 2
D)2 < 4 < 1 < 3
E)4 < 3 < 2 < 1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following could undergo a nucleophilic aromatic substitution via addition-elimination?
A)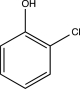
B)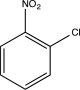
C)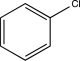
D)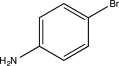
E)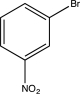
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
What arenium intermediates are formed in the ortho- and meta-substituted products of the reaction below? Draw a mechanism that leads to the formation of each intermediate,and use resonance structures to explain which would be the major product. 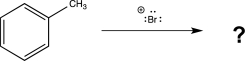


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Draw a complete,detailed mechanism for the reaction below.Label each elementary step,and indicate the rate-determining step. 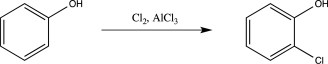


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Fill in the missing reagents to carry out the synthesis below. 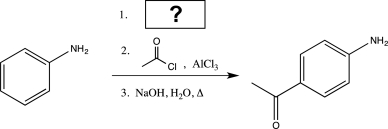
A)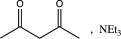
B)HNO3, H2SO4
C)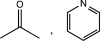
D)ZN(Hg), HCl
E)KMnO4, NaOH,

A)

B)HNO3, H2SO4
C)

D)ZN(Hg), HCl
E)KMnO4, NaOH,

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Rank the following intermediates from highest to lowest potential energy. 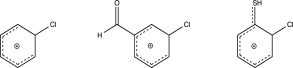


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Draw a complete,detailed mechanism for the reaction below.Also draw a resonance structure that shows why the methoxy group is an ortho/para-directing group. 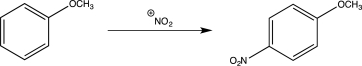


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Halogen substituents are deactivating but are ortho/para directors.Explain.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the major product of the reaction below. 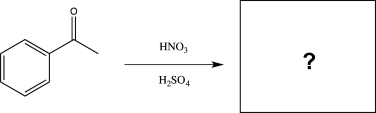


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the most stable resonance structure of the intermediate formed in the reaction below,and predict the major product(s). 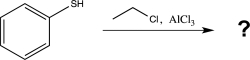


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Predict the two major products of the reaction below. 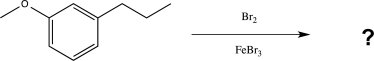


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following would be a major product of the reaction below? 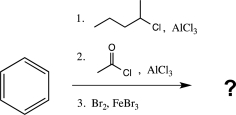
A)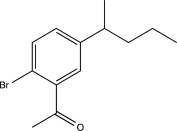
B)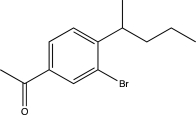
C)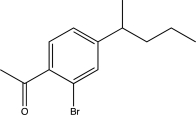
D)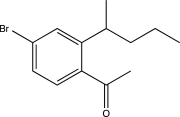
E)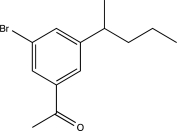

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the major product of the following reaction sequence. 
A)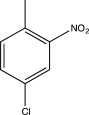
B)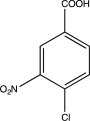
C)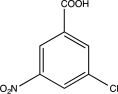
D)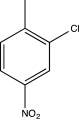
E)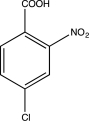

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the major product of the reaction below. 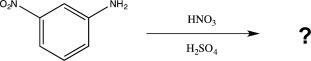
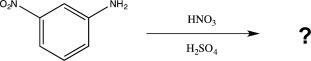

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Fill in the necessary reagent and solvent to carry out the reaction below. 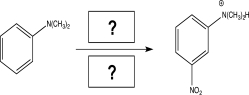


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Give an example of a monosubstituted benzene ring that would undergo bromination at a higher rate than toluene.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
A student proposes the following synthesis to produce ethylbenzene as the major product.Explain the error in this proposed synthesis,and suggest an alternative synthetic route. 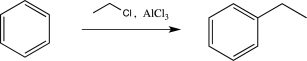


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Draw a resonance structure to explain why anisole undergoes electrophilic aromatic nitration reactions at a higher rate than benzene.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Predict the major product of the following reaction sequence. 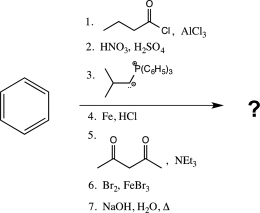
A)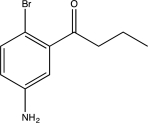
B)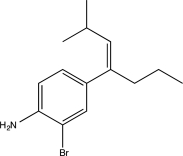
C)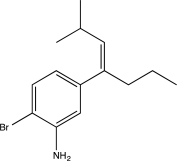
D)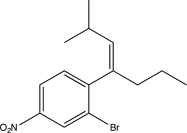
E)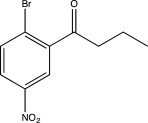

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Briefly explain why the reaction below would be unsuccessful. 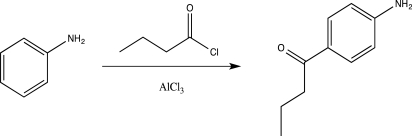


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
What is the major product of the following sequence of reactions? 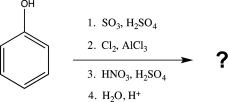
A)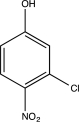
B)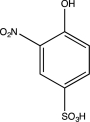
C)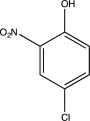
D)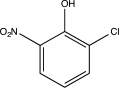
E)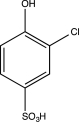

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the major product(s)of the reaction below,and draw three resonance structures for the intermediate that is formed. 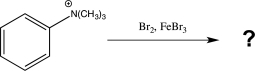


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the mechanism for the reaction below.Label each elementary step. 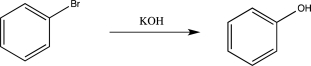


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
An amine substituent will coordinate to a Lewis acid such as AlCl3,thereby deactivating the benzene ring.If the amine is converted to an amide,however,this coordination does not take place.Briefly explain why this is so,using a resonance structure to illustrate your answer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Draw all possible isomers that could be formed in the reaction below,and predict the major product. 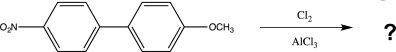


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Draw a complete,detailed mechanism for the reaction below,and predict the product.Draw the structure of the most stable intermediate. 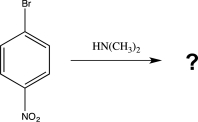


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Show how you would synthesize the compound below from benzene. 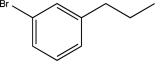


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the mechanism for the reaction below,including the structure of the most stable intermediate,and predict the major product.Label each step,and indicate the rate-determining step. 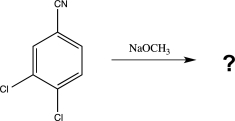


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Arrange the following in order of increasing reaction rate with respect to nucleophilic aromatic substitution by methylamine. 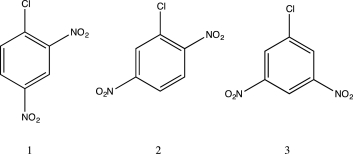


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Devise a synthesis to carry out the following transformation. 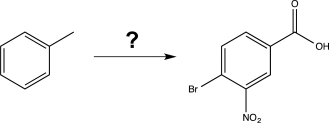


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Fill in the missing starting material for the synthesis below. 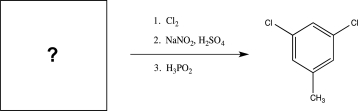


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Fill in the intermediates and reagents to complete the synthetic scheme below. 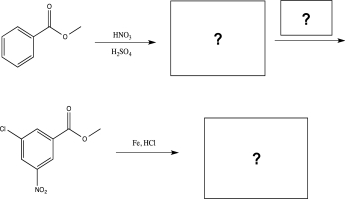


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


