Deck 28: Pericyclic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 28: Pericyclic Reactions
1
In the allyl radical,which π molecular orbital is singly occupied?
A)the bonding π molecular orbital
B)the nonbonding π molecular orbital
C)the antibonding π molecular orbital
D)none of the above
A)the bonding π molecular orbital
B)the nonbonding π molecular orbital
C)the antibonding π molecular orbital
D)none of the above
the nonbonding π molecular orbital
2
Which of the following reactions best describes the Diels-Alder reaction?
A)electrocyclic reaction
B)cycloaddition reaction
C)sigmatropic reaction
D)radical reaction
E)nucleophilic substitution reaction
A)electrocyclic reaction
B)cycloaddition reaction
C)sigmatropic reaction
D)radical reaction
E)nucleophilic substitution reaction
cycloaddition reaction
3
Which of the following statements best describes the theory of Conservation of Orbital Symmetry?
A)Molecular orbital of the transition state must be similar to that of the reactant.
B)Molecular orbital of the transition state must be similar to that of the product.
C)Only s orbitals from reactants and products are utilized.
D)Molecular orbitals of reactant and product must have similar symmetry.
E)Molecular orbitals of reactant and product must have different symmetry.
A)Molecular orbital of the transition state must be similar to that of the reactant.
B)Molecular orbital of the transition state must be similar to that of the product.
C)Only s orbitals from reactants and products are utilized.
D)Molecular orbitals of reactant and product must have similar symmetry.
E)Molecular orbitals of reactant and product must have different symmetry.
Molecular orbitals of reactant and product must have similar symmetry.
4
Which of the following molecular orbitals are formed from subtractive overlap of atomic orbitals?
A)antibonding orbitals
B)out-of-phase orbitals
C)bonding orbitals
D)A and B
E)B and C
A)antibonding orbitals
B)out-of-phase orbitals
C)bonding orbitals
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
Describe an electrocyclic reaction.
A)The reactant has lost a sigma bond,the product has a new sigma bond,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.
A)The reactant has lost a sigma bond,the product has a new sigma bond,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
How many nodes does the highest energy molecular orbital of 1,3,5,7-octatetraene have?
A)4
B)5
C)6
D)8
E)10
A)4
B)5
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following sets of atomic orbitals form an asymmetric molecular orbital? 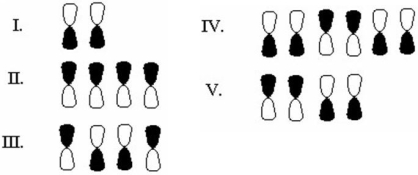
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
Describe a cycloaddition reaction.
A)The reactant has lost a sigma bond,the product has a new sigma bond,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.
A)The reactant has lost a sigma bond,the product has a new sigma bond,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
What is meant by frontier orbitals?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
Describe a sigmatropic rearrangement.
A)A sigma bond in the reactant has moved to a different location in the product,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.
A)A sigma bond in the reactant has moved to a different location in the product,and the pi bonds have rearranged.
B)The product contains a new ring,with two less sigma bonds,and two more pi bonds than the starting material.
C)The product contains a new ring,with two more sigma bonds,and two less pi bonds than the starting material.
D)The product contains a new ring,with one less sigma bond,and one more pi bond than the starting material.
E)The product contains a new ring,with one more sigma bond,and one less pi bond than the starting material.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
What are the three most common pericyclic reactions? Give examples.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
How many π molecular orbitals does 1,3,5,7-octatetraene have?
A)2
B)4
C)6
D)8
E)10
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
What does LCAO stand for?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements concerning the selection rules for pericyclic reactions is correct?
A)If TE describes the reaction,then the outcome is given by AC.
B)If PO describes the reaction,then the outcome is given by AC.
C)If TO describes the reaction,then the outcome is given by AC.
D)both A and B
E)none of the above
A)If TE describes the reaction,then the outcome is given by AC.
B)If PO describes the reaction,then the outcome is given by AC.
C)If TO describes the reaction,then the outcome is given by AC.
D)both A and B
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecular orbitals is produced when in-phase atomic orbitals overlap?
A)a bonding molecular orbital
B)an antibonding molecular orbital
C)a nonbonding molecular orbital
D)an out-of-phase molecular orbital
E)an excited energy state of molecular orbital
A)a bonding molecular orbital
B)an antibonding molecular orbital
C)a nonbonding molecular orbital
D)an out-of-phase molecular orbital
E)an excited energy state of molecular orbital

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
Through what type of single pericyclic reaction did the reaction shown below proceed? ![<strong>Through what type of single pericyclic reaction did the reaction shown below proceed? </strong> A)[1,2] sigmatropic hydrogen migration B)[1,3] sigmatropic hydrogen migration C)[1,4] sigmatropic hydrogen migration D)[1,5] sigmatropic hydrogen migration E)none of the above](https://storage.examlex.com/TB1831/11ea8a1c_2dbd_ebce_b657_070b56f04203_TB1831_00.jpg)
A)[1,2] sigmatropic hydrogen migration
B)[1,3] sigmatropic hydrogen migration
C)[1,4] sigmatropic hydrogen migration
D)[1,5] sigmatropic hydrogen migration
E)none of the above
![<strong>Through what type of single pericyclic reaction did the reaction shown below proceed? </strong> A)[1,2] sigmatropic hydrogen migration B)[1,3] sigmatropic hydrogen migration C)[1,4] sigmatropic hydrogen migration D)[1,5] sigmatropic hydrogen migration E)none of the above](https://storage.examlex.com/TB1831/11ea8a1c_2dbd_ebce_b657_070b56f04203_TB1831_00.jpg)
A)[1,2] sigmatropic hydrogen migration
B)[1,3] sigmatropic hydrogen migration
C)[1,4] sigmatropic hydrogen migration
D)[1,5] sigmatropic hydrogen migration
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
How many molecular orbitals are produced from the overlap of four p atomic orbitals?
A)2
B)8
C)4
D)16
E)1
A)2
B)8
C)4
D)16
E)1

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
Show the mechanism for each of the following pericyclic reactions. 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
How many electrons are present in the nonbonding π molecular orbital of the allyl anion?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
Indicate whether each of the following reactions is an electrocyclic reaction,a cycloaddition reaction,or a sigmatropic rearrangement. 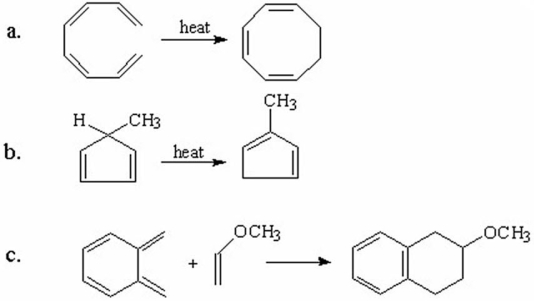


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
Under thermal conditions,(2E,4Z)-hexadiene undergoes a ________ ring closure to yield ________.
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
Under thermal conditions,(2E,4E)-hexadiene undergoes a ________ ring closure to yield ________.
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
Give the product(s)for the following reaction. 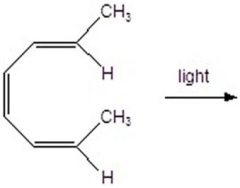
A)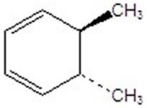
B)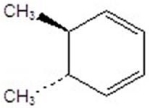
C)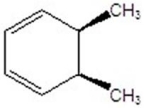
D)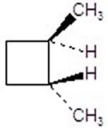
E)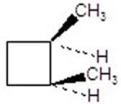

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major product of the following reaction? 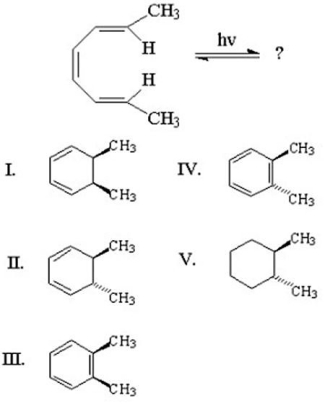
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
Give the product(s)for the following reaction.You may choose more than one answer. 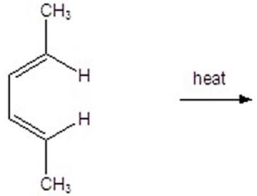
A)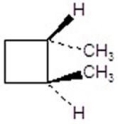
B)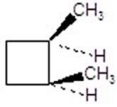
C)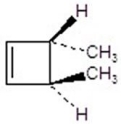
D)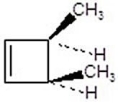
E)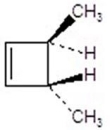

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
Which molecular orbital is higher in energy: the in-phase or out-of-phase overlap of atomic orbitals?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
(a)Under thermal conditions,will ring closure of (2E,4Z,6Z,8E)-decatetraene be conrotatory or disrotatory? Explain.
(b)Will the product be cis or trans? Explain
(b)Will the product be cis or trans? Explain

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
How many nodes would you expect from the following atomic orbital overlap? 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements about the product of the electrocyclic ring closure below is correct? 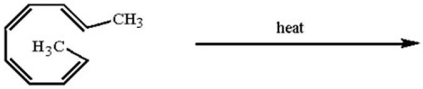
A)The methyl groups are trans in the product.
B)The product arose from a stereospecific,conrotatory ring closure.
C)The product mixture is optically active.
D)both B and C
E)all of the above
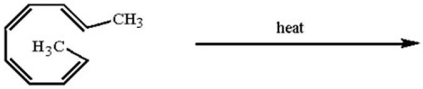
A)The methyl groups are trans in the product.
B)The product arose from a stereospecific,conrotatory ring closure.
C)The product mixture is optically active.
D)both B and C
E)all of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
Explain what a node is and how it is formed.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements concerning electrocyclic reactions is correct?
A)The ground state HOMO of a compound with an even number of conjugated double bonds is asymmetric.
B)The allowed mode of ring closure under thermal conditions for a molecule containing an even number of conjugated double bonds is disrotatory.
C)The allowed mode of ring closure under photochemical conditions for a molecule containing an odd number of conjugated double bonds is disrotatory.
D)both B and C
E)none of the above
A)The ground state HOMO of a compound with an even number of conjugated double bonds is asymmetric.
B)The allowed mode of ring closure under thermal conditions for a molecule containing an even number of conjugated double bonds is disrotatory.
C)The allowed mode of ring closure under photochemical conditions for a molecule containing an odd number of conjugated double bonds is disrotatory.
D)both B and C
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
Under photochemical conditions,(2E,4Z)-hexadiene undergoes a ________ ring closure to yield ________.
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
Give a representation of the bonding π molecular orbital of the allyl cation.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
Electrocyclic reactions are reversible.The cyclic compound is favored for electrocyclic reactions that form six-membered rings,whereas the open-chain compound is favored for reactions that form four-membered rings.Explain thoroughly in terms of the enthalpy changes that are occurring.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
Under photochemical conditions,(2E,4E)-hexadiene undergoes a ________ ring closure to yield ________.
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene
A)conrotatory,cis-3,4-dimethylcyclobutene
B)conrotatory,trans-3,4-dimethylcyclobutene
C)disrotatory,cis-3,4-dimethylcyclobutene
D)disrotatory,trans-3,4-dimethylcyclobutene

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
Give a representation of the antibonding π molecular orbital of the allyl anion.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Draw the structure of the major organic product of the following reaction.Is the product chiral or achiral? 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
Give the product(s)for the following reaction.You may choose more than one answer. 
A)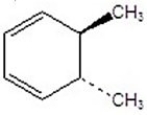
B)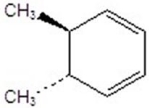
C)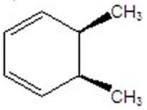
D)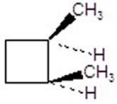
E)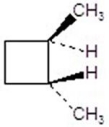

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
What is the major product of the following reaction? 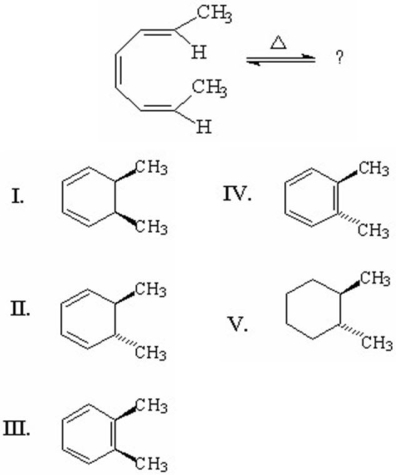
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the π molecular orbitals of pentadienyl cation and answer the following questions.Please refer to the lowest π MO as ψ1.
a)How many π molecular orbitals of pentadienyl cation are bonding?
b)Which π molecular orbital is the HOMO?
c)Which π molecular orbitals are asymmetric with respect to a mirror plane perpendicular to the molecular plane?
a)How many π molecular orbitals of pentadienyl cation are bonding?
b)Which π molecular orbital is the HOMO?
c)Which π molecular orbitals are asymmetric with respect to a mirror plane perpendicular to the molecular plane?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
What is the major product of the following reaction? 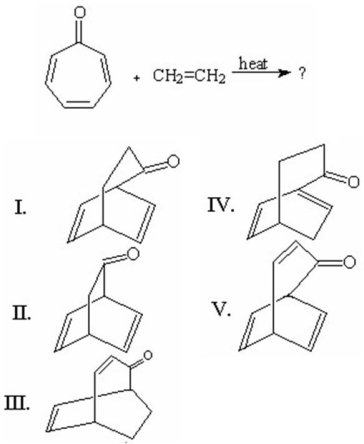
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
Give the product for the following reaction. 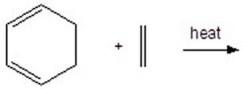
A)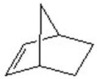
B)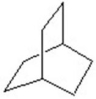
C)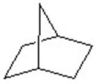
D)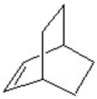
E)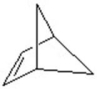
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the possible thermal [4+4] cycloaddition of two molecules of 1,3-butadiene to generate cycloocta-1,5-diene.Show the HOMO/LUMO interaction which would result,and use this interaction to predict whether the proposed cycloaddition could occur.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
Give the product for the following thermal reaction. 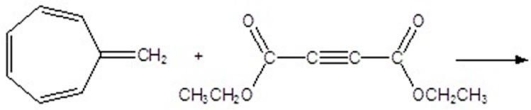
A)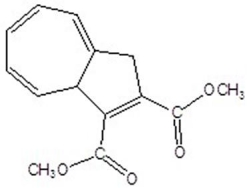
B)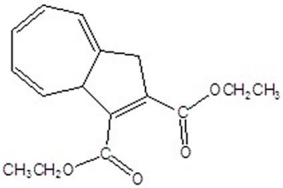
C)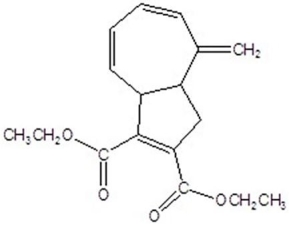
D)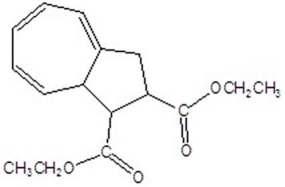
E)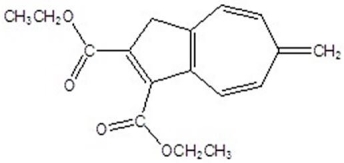

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements best describes the term suprafacial?
A)formation of two sigma bonds from the same side of the pi system
B)formation of two pi bonds from the same side of the sigma system
C)formation of two sigma bonds from opposite sides of the pi system
D)formation of two pi bonds from opposite sides of the sigma system
E)an anti cycloaddition
A)formation of two sigma bonds from the same side of the pi system
B)formation of two pi bonds from the same side of the sigma system
C)formation of two sigma bonds from opposite sides of the pi system
D)formation of two pi bonds from opposite sides of the sigma system
E)an anti cycloaddition

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
Explain why the following (2+2)cycloaddition reaction would work under photochemical conditions but not under thermal conditions. 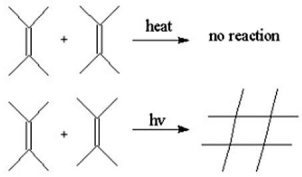


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
Is the thermal [4+2] cycloaddition between allylanion and ethylene an allowed one? To answer,draw the HOMO of allyl anion and the LUMO of ethylene,and comment on the symmetry match of the two.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
When an allyl anion undergoes a cycloaddition under thermal conditions with ethylene (bonds forming at the ends of the π-systems),the cycloaddition is described as
A)[2+2] and suprafacial.
B)[2+4] and suprafacial.
C)[2+2] and antarafacial.
D)[2+4] and antarafacial.
E)[2+3] and antarafacial.
A)[2+2] and suprafacial.
B)[2+4] and suprafacial.
C)[2+2] and antarafacial.
D)[2+4] and antarafacial.
E)[2+3] and antarafacial.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
Under photochemical conditions,is an [2+2] cycloaddition suprafacial or antarafacial?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
Give the product for the following reaction. 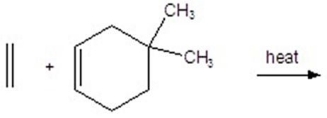
A)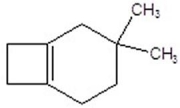
B)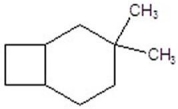
C)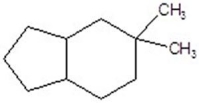
D)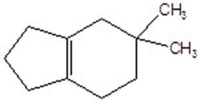
E)no reaction

A)

B)

C)

D)

E)no reaction

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
Provide the structure of the major organic product in the following reaction. 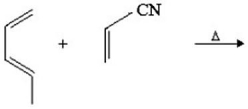


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
Why does the diene shown below fail to undergo a Diels-Alder reaction with even the most reactive dienophiles? 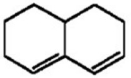


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
Under thermal conditions,is an [8+2] cycloaddition suprafacial or antarafacial?

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
What are the structures of A,B,and C? 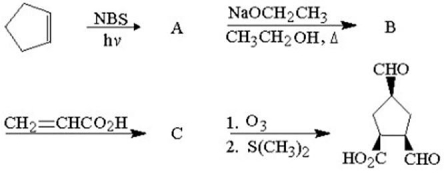


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
Give the product for the following reaction. 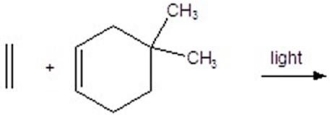
A)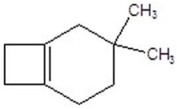
B)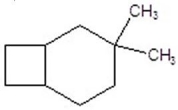
C)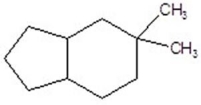
D)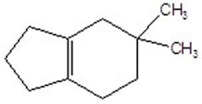
E)no reaction

A)

B)

C)

D)

E)no reaction

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
When an allyl cation undergoes a cycloaddition under photochemical conditions with ethylene (bonds forming at the ends of the π-systems),the cycloaddition is described as
A)[2+2] and suprafacial.
B)[2+4] and suprafacial.
C)[2+2] and antarafacial.
D)[2+4] and antarafacial.
E)[2+3] and antarafacial.
A)[2+2] and suprafacial.
B)[2+4] and suprafacial.
C)[2+2] and antarafacial.
D)[2+4] and antarafacial.
E)[2+3] and antarafacial.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
What diene and dienophile would react to give the product below? 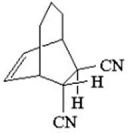


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
Give the product for the following reaction. 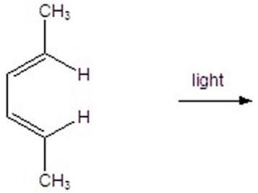
A)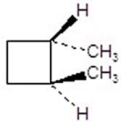
B)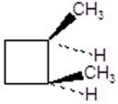
C)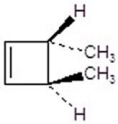
D)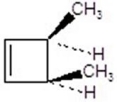
E)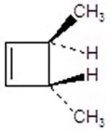

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following ring formations must involve a suprafacial bond formation?
A)four-membered ring
B)five-membered ring
C)six-membered ring
D)A and C
E)A,B and C
A)four-membered ring
B)five-membered ring
C)six-membered ring
D)A and C
E)A,B and C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following pericyclic reactions best describes the following reaction? 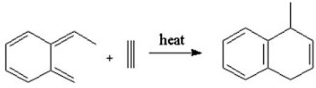
A)nucleophilic
B)electrocyclic
C)radical
D)sigmatropic
E)cycloaddition

A)nucleophilic
B)electrocyclic
C)radical
D)sigmatropic
E)cycloaddition

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
Under what conditions would a 1,3-hydrogen shift occur in a sigmatropic rearrangement?
A)thermal conditions
B)photochemical conditions
C)suprafacial pathway
D)A and C
E)B and C
A)thermal conditions
B)photochemical conditions
C)suprafacial pathway
D)A and C
E)B and C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the major organic product of the following Diels-Alder cycloaddition. 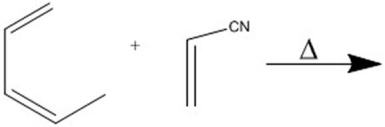


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
What is the product of the following 1,5-hydrogen shift? CH3CH  CHCH
CHCH  CD2
CD2  ?
?
A)CH2 CHCH2CH
CHCH2CH  CHD
CHD
B)CH2 CHCH
CHCH  CHCHD2
CHCHD2
C)CHD CHCH
CHCH  CHCH3
CHCH3
D)CH2 CHCH2CH2CH2D
CHCH2CH2CH2D
E)CH2 CHCH
CHCH  CHCD3
CHCD3
 CHCH
CHCH  CD2
CD2  ?
?A)CH2
 CHCH2CH
CHCH2CH  CHD
CHDB)CH2
 CHCH
CHCH  CHCHD2
CHCHD2C)CHD
 CHCH
CHCH  CHCH3
CHCH3D)CH2
 CHCH2CH2CH2D
CHCH2CH2CH2DE)CH2
 CHCH
CHCH  CHCD3
CHCD3
Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
What is the product from the following 1,3-carbon shift reaction? 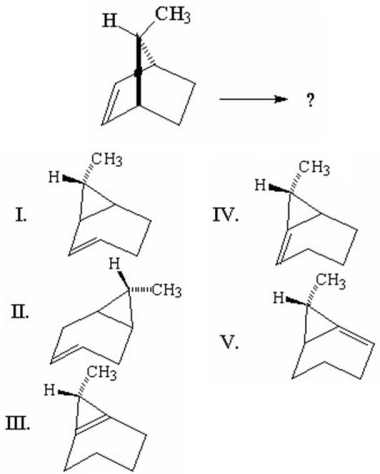
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following best describes the sigmatropic rearrangement that occurs in the reaction shown below? ![<strong>Which of the following best describes the sigmatropic rearrangement that occurs in the reaction shown below? </strong> A)[1,3] sigmatropic rearrangement B)[2,3] sigmatropic rearrangement C)[3,3] sigmatropic rearrangement D)[1,5] sigmatropic rearrangement E)[1,4] sigmatropic rearrangement](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_2886_b657_ab1d7d8d31d5_TB1831_00.jpg)
A)[1,3] sigmatropic rearrangement
B)[2,3] sigmatropic rearrangement
C)[3,3] sigmatropic rearrangement
D)[1,5] sigmatropic rearrangement
E)[1,4] sigmatropic rearrangement
![<strong>Which of the following best describes the sigmatropic rearrangement that occurs in the reaction shown below? </strong> A)[1,3] sigmatropic rearrangement B)[2,3] sigmatropic rearrangement C)[3,3] sigmatropic rearrangement D)[1,5] sigmatropic rearrangement E)[1,4] sigmatropic rearrangement](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_2886_b657_ab1d7d8d31d5_TB1831_00.jpg)
A)[1,3] sigmatropic rearrangement
B)[2,3] sigmatropic rearrangement
C)[3,3] sigmatropic rearrangement
D)[1,5] sigmatropic rearrangement
E)[1,4] sigmatropic rearrangement

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
What is the major product from the following rearrangement? 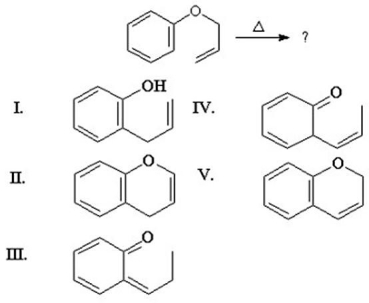
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements about carbon migrations is correct?
A)Carbon migrations never occur by a sigmatropic rearrangement.
B)Under thermal conditions,a [1,3] carbon migration that occurs suprafacially must also occur with inversion of configuration at the migrating carbon.
C)Under thermal conditions,a [1,5] carbon migration that occurs suprafacially must also occur with inversion of configuration at the migrating carbon.
D)Under thermal conditions,a carbon migration that occurs suprafacially must occur with inversion no matter the system's symmetry.
E)B,C,and D
A)Carbon migrations never occur by a sigmatropic rearrangement.
B)Under thermal conditions,a [1,3] carbon migration that occurs suprafacially must also occur with inversion of configuration at the migrating carbon.
C)Under thermal conditions,a [1,5] carbon migration that occurs suprafacially must also occur with inversion of configuration at the migrating carbon.
D)Under thermal conditions,a carbon migration that occurs suprafacially must occur with inversion no matter the system's symmetry.
E)B,C,and D

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the major organic product of the following Diels-Alder cycloaddition. 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following best explains the rearrangement when provitamin D3 rearranges to vitamin D3? ![<strong>Which of the following best explains the rearrangement when provitamin D<sub>3</sub> rearranges to vitamin D<sub>3</sub>? </strong> A)[1,7] cycloaddition rearrangement,antarafacial B)[1,7] cycloaddition rearrangement,suprafacial C)[1,7] sigmatropic rearrangement,antarafacial D)[1,7] sigmatropic rearrangement,suprafacial E)[1,7] electrocyclic reaction,suprafacial](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_4f97_b657_a754f0480875_TB1831_00.jpg)
A)[1,7] cycloaddition rearrangement,antarafacial
B)[1,7] cycloaddition rearrangement,suprafacial
C)[1,7] sigmatropic rearrangement,antarafacial
D)[1,7] sigmatropic rearrangement,suprafacial
E)[1,7] electrocyclic reaction,suprafacial
![<strong>Which of the following best explains the rearrangement when provitamin D<sub>3</sub> rearranges to vitamin D<sub>3</sub>? </strong> A)[1,7] cycloaddition rearrangement,antarafacial B)[1,7] cycloaddition rearrangement,suprafacial C)[1,7] sigmatropic rearrangement,antarafacial D)[1,7] sigmatropic rearrangement,suprafacial E)[1,7] electrocyclic reaction,suprafacial](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_4f97_b657_a754f0480875_TB1831_00.jpg)
A)[1,7] cycloaddition rearrangement,antarafacial
B)[1,7] cycloaddition rearrangement,suprafacial
C)[1,7] sigmatropic rearrangement,antarafacial
D)[1,7] sigmatropic rearrangement,suprafacial
E)[1,7] electrocyclic reaction,suprafacial

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
What is the product of the following 1,7-hydrogen shift? 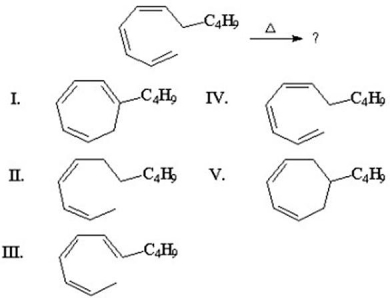
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following compounds is the most reactive dienophile in a Diels-Alder reaction with 1,3-butadiene?
A)CH2 CHOCH3
CHOCH3
B)CH2 CHCHO
CHCHO
C)CH3CH CHCH3
CHCH3
D)(CH3)2C CH2
CH2
E)CH2 CH2
CH2
A)CH2
 CHOCH3
CHOCH3B)CH2
 CHCHO
CHCHOC)CH3CH
 CHCH3
CHCH3D)(CH3)2C
 CH2
CH2E)CH2
 CH2
CH2
Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
Provide the major organic product of the following Diels-Alder cycloaddition. 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
Under what conditions would a 1,5-hydrogen shift occur in a sigmatropic rearrangement?
A)thermal conditions
B)photochemical conditions
C)suprafacial pathway
D)A and C
E)B and C
A)thermal conditions
B)photochemical conditions
C)suprafacial pathway
D)A and C
E)B and C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the major organic product of the following Diels-Alder cycloaddition. 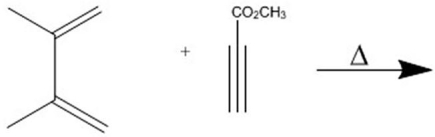


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
What is the major product of the following [3,3] sigmatropic rearrangement? ![<strong>What is the major product of the following [3,3] sigmatropic rearrangement? </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_76a8_b657_951aca177131_TB1831_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>What is the major product of the following [3,3] sigmatropic rearrangement? </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB1831/11ea8a1c_2dc6_76a8_b657_951aca177131_TB1831_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
What type of pericyclic process is occurring in the reaction below? Provide two reasons why the equilibrium lies in the direction indicated. 


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
What is the product obtained from the following [1,5] sigmatropic rearrangement? ![<strong>What is the product obtained from the following [1,5] sigmatropic rearrangement? </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB1831/11ea8a1c_2dc8_4b79_b657_0dccf7450136_TB1831_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>What is the product obtained from the following [1,5] sigmatropic rearrangement? </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB1831/11ea8a1c_2dc8_4b79_b657_0dccf7450136_TB1831_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
Provide the major organic product of the following Diels-Alder cycloaddition. 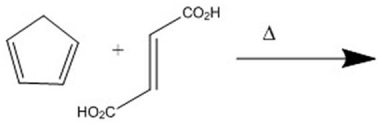


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
What is/are the product(s)of the following 1,3-hydrogen shift? 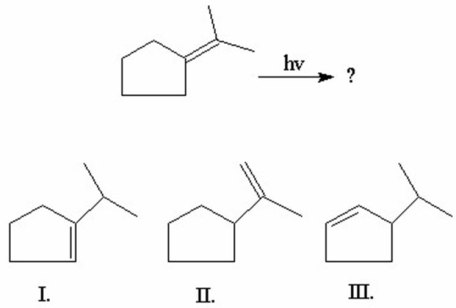
A)I
B)II
C)III
D)I and II
E)I and III

A)I
B)II
C)III
D)I and II
E)I and III

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
Show the mechanism for the following 1,3-hydrogen shift. 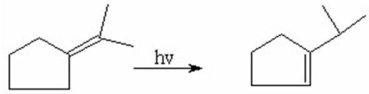


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck


