Deck 12: Radicals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/141
Play
Full screen (f)
Deck 12: Radicals
1
During the free radical chlorination of methane,which of the following reactions has the lowest collision frequency?
A)Cl ∙ + ∙ CH3 → CH3Cl
B)Cl ∙ + Cl2 → Cl2 + Cl ∙
C)∙ CH3 + CH4 → CH4 + ∙ CH3
D)∙ CH3 + Cl2 → CH3Cl + Cl ∙
A)Cl ∙ + ∙ CH3 → CH3Cl
B)Cl ∙ + Cl2 → Cl2 + Cl ∙
C)∙ CH3 + CH4 → CH4 + ∙ CH3
D)∙ CH3 + Cl2 → CH3Cl + Cl ∙
Cl ∙ + ∙ CH3 → CH3Cl
2
Consider the bond dissociation energies listed below in kcal/mol. CH3-Br 70
CH3CH2-Br 68
(CH3)2CH-Br 68
(CH3)3C-Br 65
These data show that the carbon-bromine bond is weakest when bromine is bound to a
A)methyl carbon.
B)primary carbon.
C)secondary carbon.
D)tertiary carbon.
E)quaternary carbon.
CH3CH2-Br 68
(CH3)2CH-Br 68
(CH3)3C-Br 65
These data show that the carbon-bromine bond is weakest when bromine is bound to a
A)methyl carbon.
B)primary carbon.
C)secondary carbon.
D)tertiary carbon.
E)quaternary carbon.
tertiary carbon.
3
If cyclohexane reacts with excess Cl2 at high temperature,how many distinct dichlorocyclohexane products are possible? Include all stereoisomers.
A)5
B)6
C)7
D)8
E)9
A)5
B)6
C)7
D)8
E)9
9
4
Calculate the overall ΔH° for the reaction shown given the bond dissociation energies (in kcal/mol)below: (CH3)3C-H + Cl-Cl → (CH3)3C-Cl + H-Cl
91 58 78.5 103
A)+181.5 kcal/mole
B)+58.0 kcal/mole
C)+32.5 kcal/mole
D)-32.5 kcal/mole
E)-57.5 kcal/mole
91 58 78.5 103
A)+181.5 kcal/mole
B)+58.0 kcal/mole
C)+32.5 kcal/mole
D)-32.5 kcal/mole
E)-57.5 kcal/mole

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a chain propagation step in the free radical chlorination of methane?
A)CH4 + Cl ∙ → ∙ CH3 + HCl
B)Cl2 → 2 Cl ∙
C)Cl ∙ + ∙ CH3 → CH3Cl
D)∙ CH3 + CH4 → CH4 + ∙CH3
A)CH4 + Cl ∙ → ∙ CH3 + HCl
B)Cl2 → 2 Cl ∙
C)Cl ∙ + ∙ CH3 → CH3Cl
D)∙ CH3 + CH4 → CH4 + ∙CH3

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
6
How many distinct dichlorination products can result when isobutane is subjected to free radical chlorination?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
7
The reaction Br2 + CH3Br → CH2Br2 + HBr was carried out.Which of the following mechanism steps is productive,but relatively unlikely to occur?
A)Br ∙ + CH3Br → HBr + ∙ CH2Br
B)Br ∙ + ∙ CH2Br → CH2Br2
C)Br ∙ + Br2 → Br2 + Br ∙
D)Br ∙ + ∙ CH3 → CH3Br
A)Br ∙ + CH3Br → HBr + ∙ CH2Br
B)Br ∙ + ∙ CH2Br → CH2Br2
C)Br ∙ + Br2 → Br2 + Br ∙
D)Br ∙ + ∙ CH3 → CH3Br

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the initiation step for the monobromination of cyclohexane? 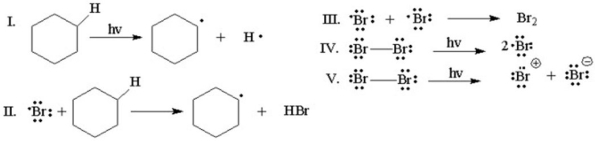
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following products result from the disproportionation reaction between two propyl radicals? 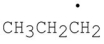
A)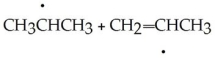
B)CH3CH2CH3 + CH3CHCH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH3 + CH2 CHCH3
CHCH3
E)CH2 C
C  CH2 + CH3CH2CH3
CH2 + CH3CH2CH3
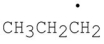
A)
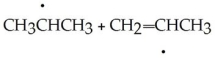
B)CH3CH2CH3 + CH3CHCH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH3 + CH2
 CHCH3
CHCH3E)CH2
 C
C  CH2 + CH3CH2CH3
CH2 + CH3CH2CH3
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
10
The major type of reactions that alkanes undergo is
A)electrophilic substitution reactions.
B)electrophilic addition reactions.
C)free radical substitution reactions.
D)free radical addition reactions.
E)nucleophilic substitution reactions.
A)electrophilic substitution reactions.
B)electrophilic addition reactions.
C)free radical substitution reactions.
D)free radical addition reactions.
E)nucleophilic substitution reactions.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the rate-determining step for the monobromination of cyclohexane? 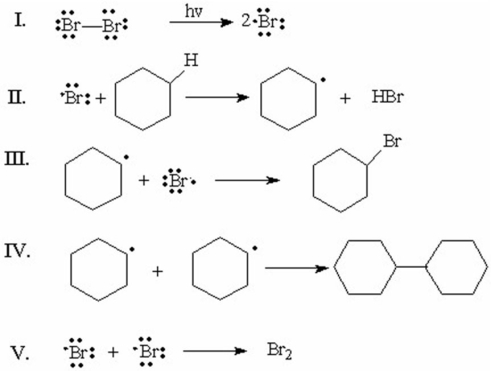
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following does not occur in a propagation step in the free radical bromination of ethane to form bromoethane?
A)a C-H bond breaks
B)a Br-H bond forms
C)a C-Br bond forms
D)a Br-Br bond breaks
E)a C-C bond breaks
A)a C-H bond breaks
B)a Br-H bond forms
C)a C-Br bond forms
D)a Br-Br bond breaks
E)a C-C bond breaks

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
13
The reaction Br2 + CH3Br → CH2Br2 + HBr was carried out.Which of the following mechanism steps is both productive and relatively likely to occur?
A)Br ∙ + ∙ CH2Br → CH2Br2
B)Br ∙ + ∙ CH3 → CH3Br
C)Br ∙ + Br2 → Br2 + Br ∙
D)Br ∙ + CH3Br → HBr + ∙ CH2Br
A)Br ∙ + ∙ CH2Br → CH2Br2
B)Br ∙ + ∙ CH3 → CH3Br
C)Br ∙ + Br2 → Br2 + Br ∙
D)Br ∙ + CH3Br → HBr + ∙ CH2Br

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
14
Given the bond dissociation energies below (in kcal/mol),estimate the ΔH° for the propagation step (CH3)2CH∙ + Cl2 → (CH3)2CHCl + Cl∙.
CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
A)-22 kcal/mol
B)+22 kcal/mol
C)-40 kcal/mol
D)+45 kcal/mol
E)-45 kcal/mol
CH3CH2CH2-H 98
(CH3)2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3)2CH-Cl 80
A)-22 kcal/mol
B)+22 kcal/mol
C)-40 kcal/mol
D)+45 kcal/mol
E)-45 kcal/mol

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
15
How many products are formed from the monochlorination of ethylcyclohexane? Ignore stereoisomers. 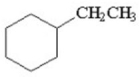
A)6
B)8
C)5
D)9
E)11

A)6
B)8
C)5
D)9
E)11

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following reactions is a termination step in the free radical chlorination of methane?
A)Cl2 + Cl ∙ → Cl ∙ + Cl2
B)Cl2 → 2 Cl ∙
C)∙ CH3 + Cl ∙ → CH3Cl
D)CH4 + Cl ∙ → HCl + ∙ CH3
A)Cl2 + Cl ∙ → Cl ∙ + Cl2
B)Cl2 → 2 Cl ∙
C)∙ CH3 + Cl ∙ → CH3Cl
D)CH4 + Cl ∙ → HCl + ∙ CH3

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
17
Explain why alkanes are generally considered unreactive compounds.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
18
Why are alkanes so unreactive?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
19
What is the major product of the following reaction? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
20
How many dichlorinated products,including stereoisomers,can be isolated when (S)-2-chlorobutane reacts with Cl2/hv? 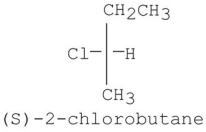
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
21
What C5H12 isomer will give only a single monochlorination product?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
22
Name the two brominated products which result when 2,3-dimethyl-2-butene reacts with NBS.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
23
Species with unpaired electrons are called ________.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not an intermediate or product in the reaction of 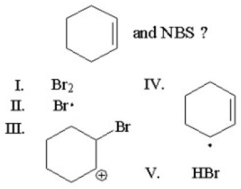
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
25
Given the bond dissociation energies below (in kcal/mol),calculate the overall ΔH° for the following reaction:
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70
(CH3)3CH + Br2 → (CH3)3CBr + HBr
(CH3)3C-H 91
(CH3)3C-Br 65
Br-Br 46
H-Br 88
CH3-Br 70

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
26
How many monochlorinated products would be obtained from 2-methylbutane? Show the structures and give their IUPAC names.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
27
How many electrons are contained in the p orbital of the methyl radical?
A)zero
B)one
C)two
D)three
A)zero
B)one
C)two
D)three

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
28
Write a detailed,stepwise mechanism for the following reaction. 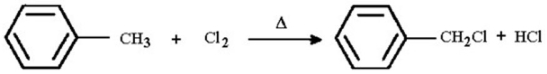
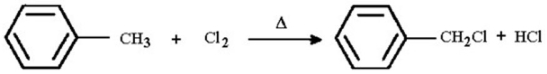

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
29
When light is shown on a mixture of chlorine and chloromethane,carbon tetrachloride is one of the components of the final reaction mixture.Propose a series of mechanistic steps which explain this observation.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major product of the following reaction? 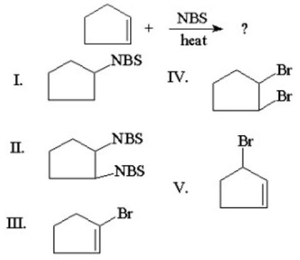
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following most nearly describes the geometry of the methyl radical?
A)trigonal pyramid,bond angle 109.5°
B)trigonal pyramid,bond angle 120°
C)trigonal planar,bond angle 109.5°
D)trigonal planar,bond angle 120°
A)trigonal pyramid,bond angle 109.5°
B)trigonal pyramid,bond angle 120°
C)trigonal planar,bond angle 109.5°
D)trigonal planar,bond angle 120°

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
32
Chlorination of methane can result in a mixture of chlorinated products.What experimental conditions should be used to favor the production of chloromethane over the other chlorinated products?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
33
Identify as initiation,propagation,or termination. 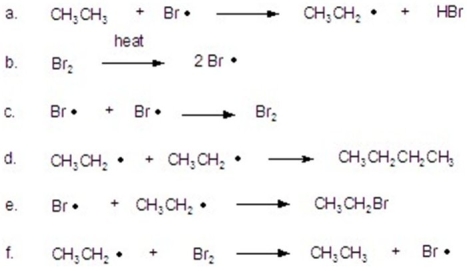
A)initiation = b; propagation = a,c,g; termination = d,e,f
B)initiation = c,d,e; propagation = b; termination = a,f
C)initiation = b; propagation = c,d,e; termination = a,f
D)initiation = b; propagation = a,f; termination = c,d,e
E)initiation = a,f; propagation = c,d,e; termination = b

A)initiation = b; propagation = a,c,g; termination = d,e,f
B)initiation = c,d,e; propagation = b; termination = a,f
C)initiation = b; propagation = c,d,e; termination = a,f
D)initiation = b; propagation = a,f; termination = c,d,e
E)initiation = a,f; propagation = c,d,e; termination = b

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following monobromination reaction,then answer the following questions. 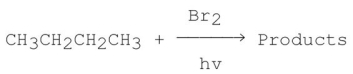 a)Give the structures and the IUPAC names for the products.
a)Give the structures and the IUPAC names for the products.
b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies: d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
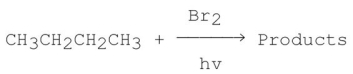 a)Give the structures and the IUPAC names for the products.
a)Give the structures and the IUPAC names for the products.b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:
 d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the reaction: CH3CH2 ∙ + Br2 → CH3CH2Br + Br ∙.Given that this reaction has an activation energy of +6 kcal/mol and a ΔH° of -22 kcal/mol,sketch a reaction-energy profile for this reaction.Label the axes and show Ea and ΔH° on your drawing.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
36
A hydrocarbon with molecular formula C5H12 is subjected to free radical chlorination conditions and only one monochlorinated product resulted.Provide the structure of this product.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
37
Write an equation to describe the initiation step in the chlorination of methane.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
38
An alkane with the molecular formula C5H10 forms only one monochlorinated product when heated with Cl2/hv.Give the structure and the IUPAC name for this alkane.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the most stable radical? 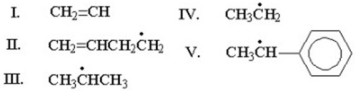
A)I
B)II
C)III
D)IV
E)V
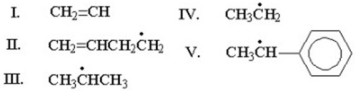
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the free radicals (I-III)shown below in order of decreasing stability (i.e.,from most stable to least stable). 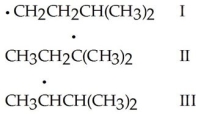
A)I > III > II
B)II > III > I
C)I > II > III
D)II > I > III
E)III > II > I
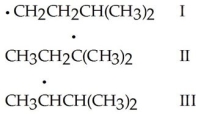
A)I > III > II
B)II > III > I
C)I > II > III
D)II > I > III
E)III > II > I

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the percentage of 1-chloro-2-methylbutane in the following reaction. 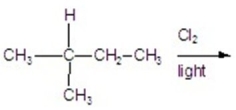
A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
42
How many distinct alkyl chlorides can be obtained from monochlorination of 2,3-dimethylpentane?
A)5
B)8
C)10
D)12
E)16
A)5
B)8
C)10
D)12
E)16

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
43
Which H in the molecule below is removed to generate the most stable carbon radical? Show the structure of this radical. 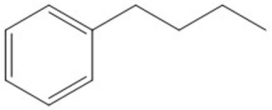


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
44
Name each distinct alkyl chloride (including stereoisomers)that can be generated from monochlorination of 2,2-dimethylbutane.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the theoretical percent yields of the four monochlorinated products that result when 2-methylbutane is subjected to free radical chlorination.Assume that the relative ease of hydrogen abstraction in the chlorination process is 5 for 3°; 3.8 for 2°; and 1 for 1° hydrogens.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
46
When Br ∙ reacts with 1-butene (CH3CH2CH  CH2),the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical.Draw the major resonance contributing forms of this radical.
CH2),the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical.Draw the major resonance contributing forms of this radical.
 CH2),the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical.Draw the major resonance contributing forms of this radical.
CH2),the hydrogen atom which is preferentially abstracted is the one which produces a resonance stabilized radical.Draw the major resonance contributing forms of this radical.
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
47
Give the structure of the free radical intermediate for each product in a previous problem.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the percentage of 2-chloro-3-methylbutane in the following reaction. 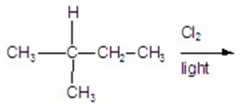
A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
49
When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C,the relative reactivity of the 1°: 2° :3° hydrogens is approximately 1: 82: 1600.Estimate the amount of each monobromination product.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
50
When butane undergoes free radical bromination,the product mixture contains 98% 2-bromobutane and 2% 1-bromobutane.How many times more susceptible to hydrogen atom abstraction is a secondary hydrogen in butane than is a primary hydrogen?
A)100
B)73.5
C)50
D)8)7
E)1)5
A)100
B)73.5
C)50
D)8)7
E)1)5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
51
An unknown sample is suspected of being either ethane or isobutane.How would you distinguish between the two alkanes?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the percentage of 2-chloro-3,4-dimethylheptane formed in the following reaction. 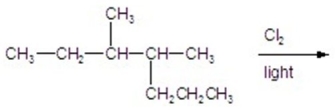
A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the percentage of 1-chloro-3-methylbutane in the following reaction. 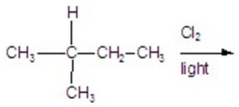
A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
54
What is the relative reactivity of 2° vs 1° hydrogens in the free radical bromination of n-butane if the ratio of 1-bromobutane to 2-bromobutane formed is 7:93?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
55
List the following radicals in order of increasing stability (ie,from least stable to most stable).
(CH3)3C ∙,CH2
 CHCH2 ∙,CH3CH2 ∙,CH3 ∙,(CH3)2CH ∙
CHCH2 ∙,CH3CH2 ∙,CH3 ∙,(CH3)2CH ∙
(CH3)3C ∙,CH2
 CHCH2 ∙,CH3CH2 ∙,CH3 ∙,(CH3)2CH ∙
CHCH2 ∙,CH3CH2 ∙,CH3 ∙,(CH3)2CH ∙
Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the percentage of 3-chloro-3,4-dimethylheptane formed in the following reaction. 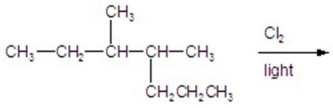
A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the percentage of 1-chloro-3,4-dimethylheptane formed in the following reaction. 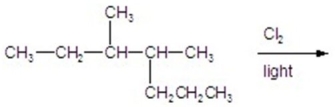
A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

A)6)70
B)11.16
C)16.96
D)22.32
E)26.80

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
58
Calculate the percentage of 2-chloro-2-methyl butane in the following reaction. 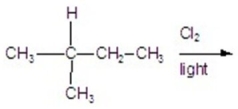
A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

A)11.56%
B)27.77%
C)23.14%
D)35.18%
E)13.88%

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the hydrogen that will react the fastest in a radical halogenation reaction. 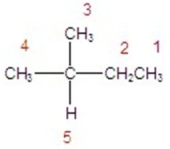
A)hydrogen 1
B)hydrogen 2
C)hydrogen 3
D)hydrogen 4
E)hydrogen 5

A)hydrogen 1
B)hydrogen 2
C)hydrogen 3
D)hydrogen 4
E)hydrogen 5

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
60
The following molecule contains how many 1°,2°,and 3° hydrogens? 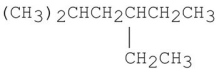
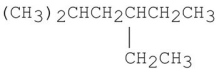

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
61
The major monobrominated product which results when methylcyclohexane is subjected to free radical bromination is
A)a primary bromide.
B)a secondary bromide.
C)a tertiary bromide.
D)a quaternary bromide.
E)bromomethane.
A)a primary bromide.
B)a secondary bromide.
C)a tertiary bromide.
D)a quaternary bromide.
E)bromomethane.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the major organic product of the reaction below. 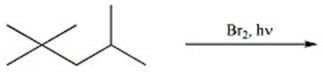


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the major monobromination product in the following reaction.
(CH3)3CCH2CH3 + Br2
(CH3)3CCH2CH3 + Br2
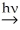

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is true about the halogenation of methane,CH4?
A)The activation energy for F ∙ + CH4 → ∙ CH3 + HF is larger than any other halogen.
B)O2 slows these reactions by inhibiting the termination step.
C)The activation energy for I ∙ + CH4 → ∙ CH3 + HI is relatively small.
D)The reaction must take place in the liquid state.
E)I2 is unreactive because the activation energy for I ∙ + CH4 → ∙ CH3 + HI is relatively large.
A)The activation energy for F ∙ + CH4 → ∙ CH3 + HF is larger than any other halogen.
B)O2 slows these reactions by inhibiting the termination step.
C)The activation energy for I ∙ + CH4 → ∙ CH3 + HI is relatively small.
D)The reaction must take place in the liquid state.
E)I2 is unreactive because the activation energy for I ∙ + CH4 → ∙ CH3 + HI is relatively large.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
65
Explain the regioselectivity observed in the radical addition of HBr to 2-methylpropene.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the best synthesis of 1,1-dibromopropane? 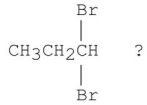
A)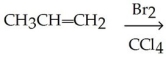
B)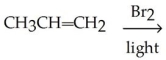
C)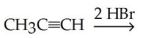
D)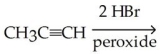
E)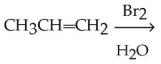

A)
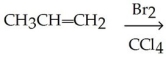
B)
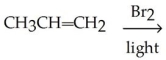
C)
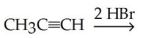
D)
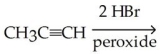
E)
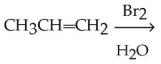

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the major organic product of the reaction shown below. 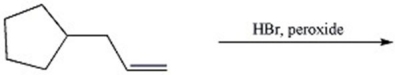
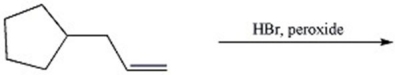

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the halogens below undergoes free radical halogenation with ethane most rapidly?
A)fluorine
B)chlorine
C)iodine
D)bromine
E)pyridine
A)fluorine
B)chlorine
C)iodine
D)bromine
E)pyridine

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
69
What is the major product of the following reaction? 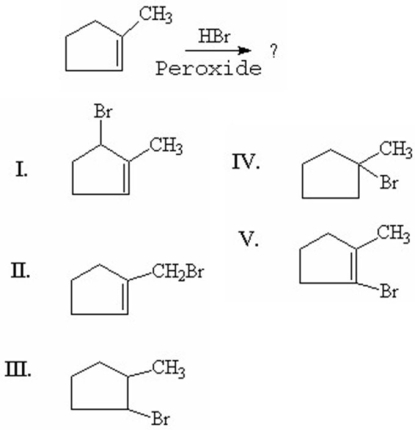
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the percentages of each product in the following reaction. 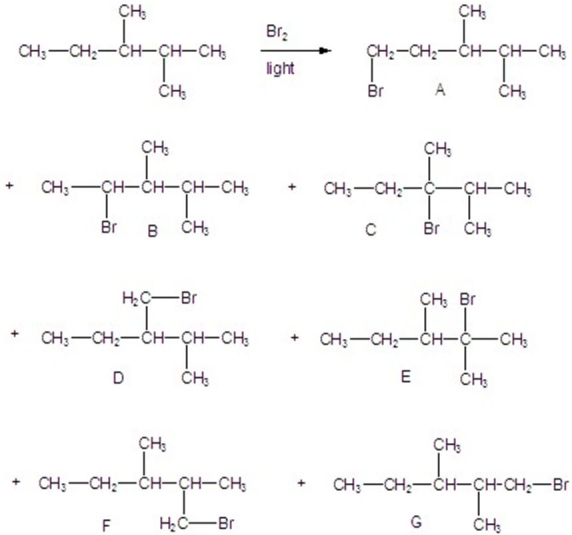


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
71
A radical mechanism is postulated to occur when cyclohexene reacts under which of the following conditions?
A)Br2,CCl4
B)H+,H2O
C)BH3∙THF
D)HBr,peroxide
E)Hg(OAc)2,H2O
A)Br2,CCl4
B)H+,H2O
C)BH3∙THF
D)HBr,peroxide
E)Hg(OAc)2,H2O

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
72
In the free radical chlorination of ethane,the step in which the Cl radical abstracts a H atom from ethane is ________ and the transition state most closely resembles ________.
A)exothermic,the reactants
B)endothermic,the reactants
C)exothermic,the products
D)endothermic,the products
E)endothermic,a carbocation
A)exothermic,the reactants
B)endothermic,the reactants
C)exothermic,the products
D)endothermic,the products
E)endothermic,a carbocation

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
73
Would chlorination or bromination of 2,5-dimethylhexane produce a greater yield of 1-halo-2,5-dimethylhexane?

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
74
Provide the structure of the major organic product of the following reaction. 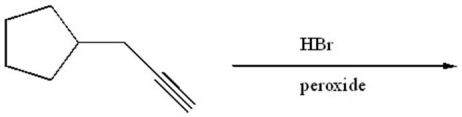


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is a step in the mechanism of the reaction shown? 
A)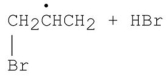
B)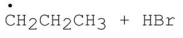
C)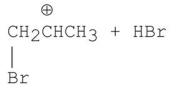
D)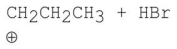
E)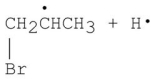

A)
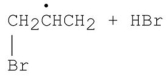
B)
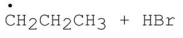
C)
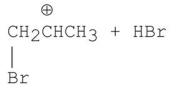
D)
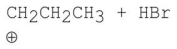
E)
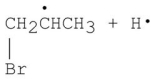

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
76
Give the best product for the following reaction. 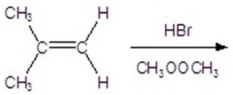
A)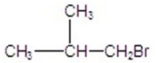
B)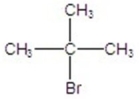
C)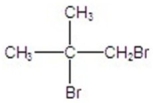
D)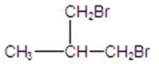
E)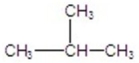

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
77
Use the Hammond Postulate to explain why free radical brominations are more selective than free radical chlorinations.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
78
What is the major product of the following reaction? 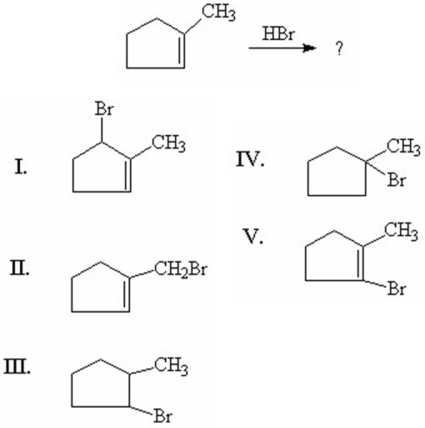
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
79
Show the propagating steps in the addition of HBr to 1-pentene in the presence of peroxide.

Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck
80
Provide the major organic product of the reaction below. 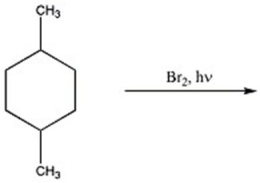


Unlock Deck
Unlock for access to all 141 flashcards in this deck.
Unlock Deck
k this deck


