Deck 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy
1
Give the m/z ratio of the molecular ion in the mass spectrum of 1-butanol.
74
2
Which of the following statements best describes the meaning of the following species [CH3CH2CH3] ∙ + ?
A)It is the molecular ion of propane.
B)It is the parent ion of propane.
C)It is the radical cation of propane.
D)The m/z value is 44.
E)all of the above
A)It is the molecular ion of propane.
B)It is the parent ion of propane.
C)It is the radical cation of propane.
D)The m/z value is 44.
E)all of the above
all of the above
3
Give the m/z ratio that corresponds to the molecular ion in the mass spectrum of 2-bromobutane.
136 and 138
4
Give the m/z ratio that corresponds to the molecular ion in the mass spectrum of 2-pentanone.
A)28
B)43
C)58
D)71
E)86
A)28
B)43
C)58
D)71
E)86

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
What does "m/z" stand for and what does it mean?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements best explains the information we can gain from mass spectrometry?
A)It allows us to determine the number of protons in a compound.
B)It allows us to determine the kinds of functional groups in a compound.
C)It allows us to determine the molecular weight and the mass of some fragments of a compound.
D)It allows us to determine the presence and nature of a carbocation in the compound.
E)It allows us to determine the presence and nature of a free radical in the compound.
A)It allows us to determine the number of protons in a compound.
B)It allows us to determine the kinds of functional groups in a compound.
C)It allows us to determine the molecular weight and the mass of some fragments of a compound.
D)It allows us to determine the presence and nature of a carbocation in the compound.
E)It allows us to determine the presence and nature of a free radical in the compound.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements best explains how a hydrocarbon can show an M+2 peak in mass spectrometry?
A)from 13C and 1H
B)from 12C and 2H
C)from a single 13C
D)from a single 3H
E)from two 13C's
A)from 13C and 1H
B)from 12C and 2H
C)from a single 13C
D)from a single 3H
E)from two 13C's

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is true about the base peak in mass spectrometry?
A)The m/z value equals the molecular weight of the compound.
B)The m/z value corresponds to a very stable carbanion.
C)It has the largest peak height in the spectrum.
D)It has the highest m/z value of all the peaks in the spectrum.
E)The base peak is assigned a relative abundance equal to that of the parent ion.
A)The m/z value equals the molecular weight of the compound.
B)The m/z value corresponds to a very stable carbanion.
C)It has the largest peak height in the spectrum.
D)It has the highest m/z value of all the peaks in the spectrum.
E)The base peak is assigned a relative abundance equal to that of the parent ion.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is true about a molecular ion?
A)It is a compound that lost a pair of electrons.
B)It is a compound that gained a pair of electrons.
C)It is a compound that gained one electron.
D)It is a compound that lost one electron.
E)It is a compound that carries a free radical and a negative charge.
A)It is a compound that lost a pair of electrons.
B)It is a compound that gained a pair of electrons.
C)It is a compound that gained one electron.
D)It is a compound that lost one electron.
E)It is a compound that carries a free radical and a negative charge.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
How could you distinguish the mass spectrum of 2,2-dimethylpropane from that of isopentane?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
What m/z value would you predict for the most stable fragment peak in the mass spectrum of 4-ethylheptane?
A)128
B)127
C)113
D)99
E)85
A)128
B)127
C)113
D)99
E)85

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not true about the M+1 peak?
A)It is one m/z unit higher than the base peak.
B)It is one m/z unit higher than the molecular ion peak.
C)It is one m/z unit higher than the parent ion peak.
D)It occurs because there is more than one naturally occurring isotope of carbon.
E)This means that the number of carbon atoms in a compound can be calculated if the relative abundance of both the M and M+1 peaks is known.
A)It is one m/z unit higher than the base peak.
B)It is one m/z unit higher than the molecular ion peak.
C)It is one m/z unit higher than the parent ion peak.
D)It occurs because there is more than one naturally occurring isotope of carbon.
E)This means that the number of carbon atoms in a compound can be calculated if the relative abundance of both the M and M+1 peaks is known.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
2-Methylhexane shows an intense peak in the mass spectrum at m/z = 43.Propose a likely structure for this fragment.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following m/z values is the molecular ion for 2-butanone? 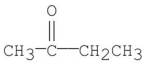
A)15
B)29
C)43
D)57
E)72
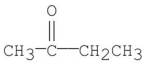
A)15
B)29
C)43
D)57
E)72

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
Give the m/z ratio that corresponds to the molecular ion in the mass spectrum of ethylpropylamine.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
Predict the molecular formula of the compound represented below based on the MS data given. 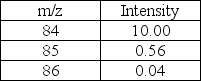
A)C6H12
B)C5H24
C)C4H6O2
D)C3H8O2
E)C5H8O

A)C6H12
B)C5H24
C)C4H6O2
D)C3H8O2
E)C5H8O

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following characterizes the unusually intense peak of alkyl chlorides in MS spectrometry?
A)M + 1 peak
B)M + 2 peak
C)base peak
D)parent peak
E)none of the above
A)M + 1 peak
B)M + 2 peak
C)base peak
D)parent peak
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecular changes is necessary for mass spectrometry to occur?
A)excitation of an electron from the ground state to higher energy state
B)change of alignment of an electron in a magnetic field
C)change of alignment of a proton in a magnetic field
D)loss of an electron
E)molecular vibration
A)excitation of an electron from the ground state to higher energy state
B)change of alignment of an electron in a magnetic field
C)change of alignment of a proton in a magnetic field
D)loss of an electron
E)molecular vibration

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not a major peak in the MS of isopentane?
A)29
B)43
C)57
D)60
E)72
A)29
B)43
C)57
D)60
E)72

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
Describe the fate of a molecule from introduction to detection in a mass spectrometer.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following m/z values is the base peak for benzyl alcohol? 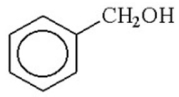
A)77
B)108
C)91
D)17
E)52

A)77
B)108
C)91
D)17
E)52

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following structures will give a base peak of 43 in mass spectrometry?
A)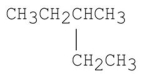
B)CH3CH2CH2CH2CH2CH3
C)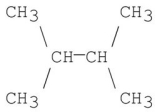
D)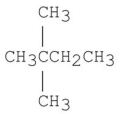
E)none of the above
A)
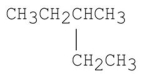
B)CH3CH2CH2CH2CH2CH3
C)

D)

E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Provide the structure of the two ions which result when the molecular ion of 2-methoxypentane undergoes fragmentation by α-cleavage.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
The mass spectrum of an unknown compound has a molecular ion peak with a relative abundance of 43.27% and an M + 1 peak with a relative abundance of 3.81%.How many carbon atoms are in the compound?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is/are true about the MS of 1-bromobutane?
A)Peaks of approximately equal intensity are observed at m/z 136 and 138.
B)The major fragmentation occurs by cleavage of the C-Br bond.
C)The most intense peak occurs at m/z 43.
D)both A and B
E)both B and C
A)Peaks of approximately equal intensity are observed at m/z 136 and 138.
B)The major fragmentation occurs by cleavage of the C-Br bond.
C)The most intense peak occurs at m/z 43.
D)both A and B
E)both B and C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
Describe the molecular ion region in the mass spectrum of CH3CH2Br.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
Provide the structure of the species which results when the molecular ion of 4-heptanone undergoes fragmentation via a McLafferty rearrangement.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
The mass spectra of alcohols often fail to exhibit detectable M peaks but instead show relatively large ________ peaks.
A)M+1
B)M+2
C)M-16
D)M-17
E)M-18
A)M+1
B)M+2
C)M-16
D)M-17
E)M-18

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
In the mass spectrum of bromoform,CHBr3,molecular ion peaks appear at m/z 250,251,252,and 253? What is the ratio of the intensities of these peaks?
A)1:1:1:1
B)1:2:2:1
C)1:3:3:1
D)2:3:3:2
E)3:2:2:3
A)1:1:1:1
B)1:2:2:1
C)1:3:3:1
D)2:3:3:2
E)3:2:2:3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds exhibits the pattern of m/z values shown below? 41,43,57,87,101,116
A)propylbromide
B)isopropyl bromide
C)sec-butyl isopropyl ether
D)2-hexanol
E)2-butanone
A)propylbromide
B)isopropyl bromide
C)sec-butyl isopropyl ether
D)2-hexanol
E)2-butanone

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
A compound shows a molecular ion peak at m/z 167.The relative intensities of the M and M+1 peaks are 50.0 and 4.4 respectively.How many carbons are in each molecule of the compound?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
An acylium ion is often produced as a fragment in the MS of which of the following class of organic compounds?
A)ketones
B)ethers
C)alkyl halides
D)alcohols
E)peroxides
A)ketones
B)ethers
C)alkyl halides
D)alcohols
E)peroxides

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
In the mass spectrum of 2-chloropropane,what is the m/z of the molecular ion peak of greatest intensity?
A)63
B)76
C)78
D)80
E)82
A)63
B)76
C)78
D)80
E)82

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
Show the m/z values of the molecular ion and 5 likely fragments for the compound ethyl ether,
CH3CH2OCH2CH3
CH3CH2OCH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following m/z values is least intense in the mass spectrum of 1-chloropropane?
A)80
B)79
C)78
D)65
E)63
A)80
B)79
C)78
D)65
E)63

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the base peak for the compound below? 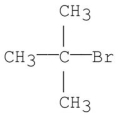
A)77
B)92
C)15
D)57
E)43

A)77
B)92
C)15
D)57
E)43

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
In the mass spectrum of 1,2-dichloroethane,what is the m/z of the molecular ion peak of greatest intensity?
A)94
B)96
C)98
D)100
E)102
A)94
B)96
C)98
D)100
E)102

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
What technique can be used to determine the molecular formula of a compound?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
Is it possible to have an M + 2 peak in mass spectrometry? Explain.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
Which compound's mass spectrum shows peaks at M,M+2,and M+4 whose abundances are in a ratio of 1:2:1?
A)cyclohexanol
B)chlorocyclohexane
C)1,2-dichlorocyclohexane
D)1-bromopentane
E)1,5-dibromopentane
A)cyclohexanol
B)chlorocyclohexane
C)1,2-dichlorocyclohexane
D)1-bromopentane
E)1,5-dibromopentane

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
Which region of the electromagnetic spectrum,IR or UV,contains photons of the higher energy?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
Ethyl acetate has the structure CH3CO2CH2CH3.Which set of m/z peaks is most consistent with this structure?
A)88,73,43
B)88,76,45
C)88,73,45
D)88,76,43
E)88,72,70,44
A)88,73,43
B)88,76,45
C)88,73,45
D)88,76,43
E)88,72,70,44

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
Give the fragment(s)that corresponds to a m/z charge of 58 for ethylpropylamine.You may choose more than one answer.
A)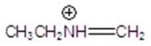
B)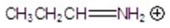
C)
D)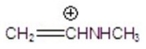
E)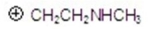
A)
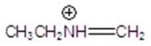
B)
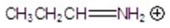
C)

D)

E)
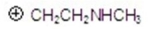

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is not true about electromagnetic radiation?
A)The velocity of light is directly proportional to the energy.
B)All molecules absorb electromagnetic radiation at some frequency.
C)Frequency is inversely proportional to wavelength.
D)Energy is directly proportional to frequency.
E)Energy is inversely proportional to wavelength.
A)The velocity of light is directly proportional to the energy.
B)All molecules absorb electromagnetic radiation at some frequency.
C)Frequency is inversely proportional to wavelength.
D)Energy is directly proportional to frequency.
E)Energy is inversely proportional to wavelength.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
Which region of the electromagnetic spectrum,IR or X-ray,is characterized by waves of lower frequency?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
Which region of the electromagnetic spectrum,radio or visible,is characterized by waves of shorter wavelength?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
Arrange the following regions of the electromagnetic spectrum in order of decreasing wavelength: microwaves,visible light,ultraviolet light,infrared radiation,X-rays.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following normally occurs in a molecule when a photon of infrared light is absorbed?
A)An electron moves to an orbital of higher potential energy.
B)A transition between allowed vibrational energy states occurs.
C)An electron changes alignment in a magnetic field.
D)The molecule gains an electron.
E)The molecule loses an electron.
A)An electron moves to an orbital of higher potential energy.
B)A transition between allowed vibrational energy states occurs.
C)An electron changes alignment in a magnetic field.
D)The molecule gains an electron.
E)The molecule loses an electron.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
When cyclopentanol is treated with chromic acid,the alcohol is oxidized to cyclopentanone.Which m/z peak is expected to be the base peak of cyclopentanone?
A)55
B)43
C)29
D)84
E)85
A)55
B)43
C)29
D)84
E)85

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
Which m/z peak is expected to be the base peak of cyclopentanol?
A)57
B)43
C)29
D)85
E)86
A)57
B)43
C)29
D)85
E)86

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
Give the ion that corresponds to a m/z ratio of 56 in a mass spectrum of 1-butanol.
A)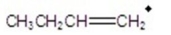
B)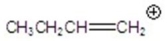
C)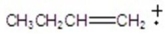
D)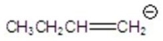
E)none of the above
A)
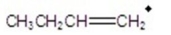
B)
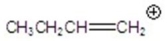
C)
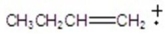
D)
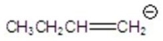
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
In the electromagnetic spectrum,________ frequencies,________ wavenumbers,and ________ wavelengths are associated with high energy.
A)high,small,long
B)low,large,short
C)low,small,short
D)high,large,short
E)high,small,short
A)high,small,long
B)low,large,short
C)low,small,short
D)high,large,short
E)high,small,short

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
Give the ion that corresponds to a m/z ratio of 58 in a mass spectrum of 2-pentanone.
A)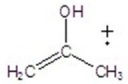
B)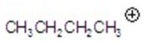
C)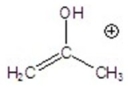
D)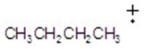
E)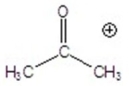
A)

B)
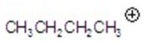
C)

D)
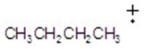
E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
Give the ion that corresponds to a m/z ratio of 43 in a mass spectrum of 2-pentanone.You may choose more than one answer.
A)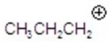
B)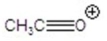
C)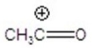
D)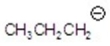
E)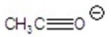
A)
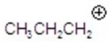
B)
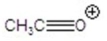
C)

D)
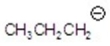
E)
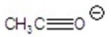

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is highest in energy per photon?
A)electromagnetic radiation with a wavenumber of 10-2 cm-1
B)electromagnetic radiation with a frequency of 107 s-1
C)electromagnetic radiation with a frequency of 105 s-1
D)electromagnetic radiation with a wavelength of 10 cm
E)electromagnetic radiation with a wavenumber of 106 cm-1
A)electromagnetic radiation with a wavenumber of 10-2 cm-1
B)electromagnetic radiation with a frequency of 107 s-1
C)electromagnetic radiation with a frequency of 105 s-1
D)electromagnetic radiation with a wavelength of 10 cm
E)electromagnetic radiation with a wavenumber of 106 cm-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
Give the ion that corresponds a m/z ratio of 31 in a mass spectrum of 1-butanol.
A)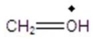
B)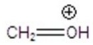
C)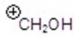
D)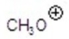
E)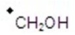
A)
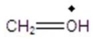
B)
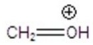
C)
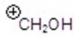
D)
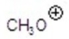
E)
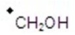

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
How many centimeters (cm)are there in one micrometer (μm)?
A)10-6
B)10-3
C)10-5
D)10-8
E)10-4
A)10-6
B)10-3
C)10-5
D)10-8
E)10-4

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
Which has the higher speed in a vacuum,ultraviolet or infrared light?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following regions of the electromagnetic spectrum has the greatest energy per photon?
A)visible
B)microwave
C)radio
D)ultraviolet
E)infrared
A)visible
B)microwave
C)radio
D)ultraviolet
E)infrared

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
An infrared wavelength of 4.48μm is equivalent to a wavenumber of ________ cm-1.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
Which molecule below has a significant band in the IR at 2220 cm-1 (medium)?
A)CH3CH2CH2OH
B)CH3C CCH2CH3
CCH2CH3
C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3
A)CH3CH2CH2OH
B)CH3C
 CCH2CH3
CCH2CH3C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following increases the absorbance of a solution of phenol in ethanol?
A)increasing the amount of phenol in the solution
B)decreasing the amount of ethanol in the solution
C)decreasing the light path through the solution
D)both A and B
E)both B and C
A)increasing the amount of phenol in the solution
B)decreasing the amount of ethanol in the solution
C)decreasing the light path through the solution
D)both A and B
E)both B and C

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following statements best explains the information we can gain from infrared spectroscopy?
A)It allows us to determine the number of protons in a compound.
B)It allows us to determine the kinds of functional groups in a compound.
C)It allows us to determine the molecular weight and the mass of some fragments of a compound.
D)It allows us to determine the presence and nature of a carbocation in the compound.
E)It allows us to determine the presence and nature of a free radical in the compound.
A)It allows us to determine the number of protons in a compound.
B)It allows us to determine the kinds of functional groups in a compound.
C)It allows us to determine the molecular weight and the mass of some fragments of a compound.
D)It allows us to determine the presence and nature of a carbocation in the compound.
E)It allows us to determine the presence and nature of a free radical in the compound.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
While cleaning the organic stockroom,a student found a bottle of ethanol labeled "Denatured with Benzene." He decided to determine the concentration of benzene in the ethanol by acquiring the UV spectrum of the liquid in a 2.0 cm cell.The spectrum exhibited an absorption band at 260 nm which was attributable to benzene,and the absorbance of this band was 0.69.In a reference source,the student found that the molar absorptivity of this benzene band in ethanol was 230 M-1cm-1.Show how the student calculated the concentration of the benzene in the ethanol.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
Which compound's carbonyl stretch occurs at the lowest wavenumber?
A)CH3CH2CH2CHO
B)CH3COCH2CH3
C)CH3CH2CONH2
D)CH3CH2CO2CH3
E)CH3CH2COCH2CH3
A)CH3CH2CH2CHO
B)CH3COCH2CH3
C)CH3CH2CONH2
D)CH3CH2CO2CH3
E)CH3CH2COCH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
Which molecule below has a significant band in the IR at 3400 cm-1 (strong and broad)?
A)CH3CH2CH2OH
B)CH3C CCH2CH3
CCH2CH3
C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3
A)CH3CH2CH2OH
B)CH3C
 CCH2CH3
CCH2CH3C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the infrared regions is considered to be the fingerprint region?
A)4000cm-1 - 1000cm-1
B)4000μm - 1000μm
C)2200μm - 1000μm
D)1000cm-1 - 400cm-1
E)1000μm - 400μm
A)4000cm-1 - 1000cm-1
B)4000μm - 1000μm
C)2200μm - 1000μm
D)1000cm-1 - 400cm-1
E)1000μm - 400μm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
At what wavenumber will the C-H stretch involving an sp carbon appear?
A)2700 cm-1
B)2900 cm-1
C)3100 cm-1
D)3300 cm-1
E)3500 cm-1
A)2700 cm-1
B)2900 cm-1
C)3100 cm-1
D)3300 cm-1
E)3500 cm-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following wavenumbers corresponds to the bond shown below? C  C
C
A)1650 cm-1
B)2100 cm-1
C)1100 cm-1
D)3300 cm-1
E)2850 cm-1
 C
CA)1650 cm-1
B)2100 cm-1
C)1100 cm-1
D)3300 cm-1
E)2850 cm-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
Which compound would be expected to show intense IR absorption at 1746 cm-1?
A)CH3CH2OCH2CH3
B)CH3CO2CH3
C)CH3CH2CCH
D)CH3CH2SCH3
A)CH3CH2OCH2CH3
B)CH3CO2CH3
C)CH3CH2CCH
D)CH3CH2SCH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following solvents is best used in infrared spectroscopy? 
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
Which compound would be expected to show intense IR absorption at 1715 cm-1?
A)CH3CH2CO2H
B)1-hexene
C)2-methylhexane
D)CH3CH2CH2NH2
A)CH3CH2CO2H
B)1-hexene
C)2-methylhexane
D)CH3CH2CH2NH2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following are considered to be bending (in-plane)vibrations?
A)scissoring and wagging
B)scissoring and twisting
C)rocking and wagging
D)rocking and twisting
E)scissoring and rocking
A)scissoring and wagging
B)scissoring and twisting
C)rocking and wagging
D)rocking and twisting
E)scissoring and rocking

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
Identify the bond with the lowest wavenumber in the IR spectrum.
A)C O
O
B)C-O
C)O-H in alcohol
D)N-H
E)C-H
A)C
 O
OB)C-O
C)O-H in alcohol
D)N-H
E)C-H

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
Identify the compound that has a formula of C5H10 and bending vibrations at 990 and 910 cm-1.
A)1-pentene
B)cis-2-pentene
C)trans-2-pentene
D)2-methyl-1-butene
E)2-methyl-2-butene
A)1-pentene
B)cis-2-pentene
C)trans-2-pentene
D)2-methyl-1-butene
E)2-methyl-2-butene

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following carbonyl groups exhibits the highest wavenumber in infrared spectroscopy? 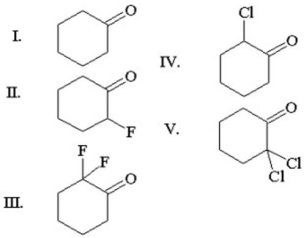
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
Which molecule below has a significant band in the 1720 - 1780 cm-1 range of its IR spectrum?
A)CH3CH2CH2OH
B)CH3C CCH2CH3
CCH2CH3
C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3
A)CH3CH2CH2OH
B)CH3C
 CCH2CH3
CCH2CH3C)CH3CH2CH(NH2)CH3
D)(CH3)3N
E)CH3CO2CH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
Which compound's carbonyl stretch occurs at the greatest wavenumber?
A)CH3CH2CH2CHO
B)CH3COCH2CH3
C)CH3CH2CONH2
D)CH3CH2CO2CH3
E)CH3CH2COCH2CH3
A)CH3CH2CH2CHO
B)CH3COCH2CH3
C)CH3CH2CONH2
D)CH3CH2CO2CH3
E)CH3CH2COCH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
Does the position of the stretch of the oxygen-hydrogen bond in alcohols depend on the concentration of the alcohol?

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is not a common bending vibration of a CH2 group?
A)flip
B)rock
C)scissor
D)twist
E)wag
A)flip
B)rock
C)scissor
D)twist
E)wag

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


