Deck 21: Amino Acids,peptides,and Proteins
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 21: Amino Acids,peptides,and Proteins
1
Which of the following amino acids has an aromatic R group?
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine
tyrosine
2
Provide the Fischer projection of L-aspartic acid.
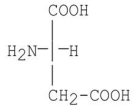
3
Explain what essential amino acids are and list all of them.
An essential amino acid is one that humans must obtain from their diet because it cannot be synthesized at all or in sufficient quantity in the body.They are valine,leucine,isoleucine,threonine,methionine,lysine,arginine,phenylalanine,histidine,and tryptophan.
4
Draw the form of L-tryptophan which is present at biological pH.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Name the following compound. 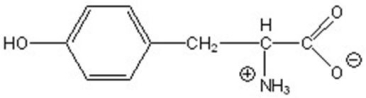
A)tyrosine
B)lysine
C)arginine
D)histidine
E)phenylalanine

A)tyrosine
B)lysine
C)arginine
D)histidine
E)phenylalanine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
Draw and name the amino acids which may be described as heterocyclic.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
Name the following compound. 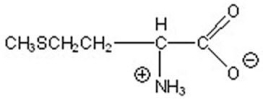
A)cysteine
B)threonine
C)methionine
D)serine
E)asparagine

A)cysteine
B)threonine
C)methionine
D)serine
E)asparagine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following amino acids is not an aromatic compound?
A)phenylalanine
B)threonine
C)histidine
D)tryptophan
E)tyrosine
A)phenylalanine
B)threonine
C)histidine
D)tryptophan
E)tyrosine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following terms best describes the side chain of valine?
A)acidic
B)basic
C)charged,polar
D)uncharged,polar
E)nonpolar
A)acidic
B)basic
C)charged,polar
D)uncharged,polar
E)nonpolar

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following amino acids has a sulfur in the R group?
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
Draw glutamate.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following amino acids has an aliphatic R group?
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine
A)serine
B)cysteine
C)asparagine
D)tyrosine
E)leucine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
In nature,which is the most commonly found isomer,L-amino acids or D-amino acids?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
Name the following compound. 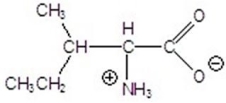
A)alanine
B)leucine
C)valine
D)glycine
E)isoleucine

A)alanine
B)leucine
C)valine
D)glycine
E)isoleucine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following amino acids has a heterocyclic R group?
A)glycine
B)threonine
C)proline
D)aspartic acid
E)arginine
A)glycine
B)threonine
C)proline
D)aspartic acid
E)arginine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
What is the net charge of arginine in a solution of pH 1.0?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following molecules is the skeletal structure of an amino acid?
A)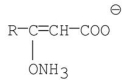
B)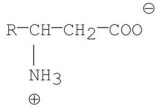
C)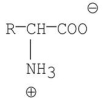
D)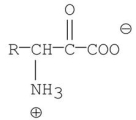
E)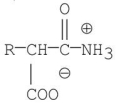
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an L-amino acid? 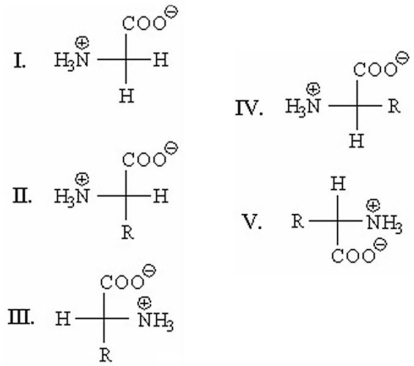
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
The α-carbon of all the amino acids is a chirality center except for
A)glycine
B)threonine
C)proline
D)aspartic acid
E)arginine
A)glycine
B)threonine
C)proline
D)aspartic acid
E)arginine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
Draw the form of L-lysine which is present at biological pH.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following amino acids will be closest to the origin when separated by thin-layer chromatography? 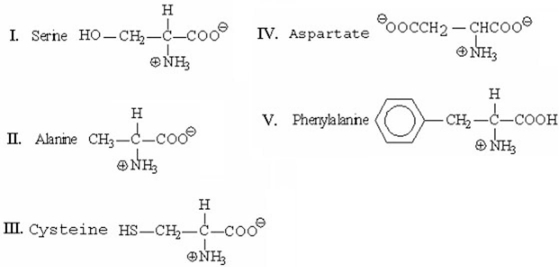
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following amino acids will be retained longest in the column when separated by cation-exchange chromatography? 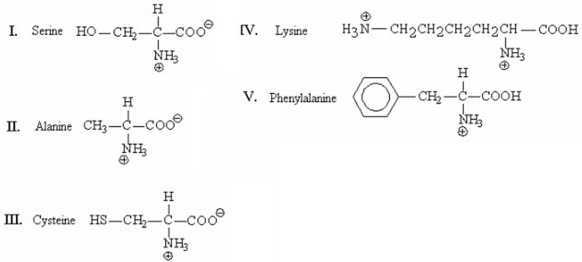
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
What is the pI of arginine? The structure and pKa values are shown below. 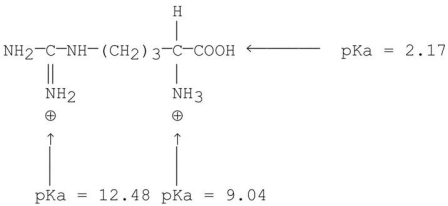
A)10.76
B)7)90
C)5)61
D)7)33
E)9)67

A)10.76
B)7)90
C)5)61
D)7)33
E)9)67

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following amino acids has a side chain with an ionizable proton and can exist in four different forms,depending on the pH of the solution? 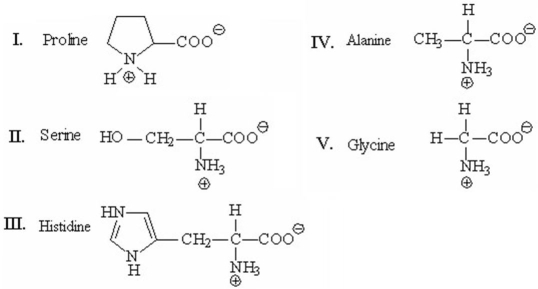
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
Draw structures for the forms of glycine present in basic,neutral,and acidic solutions.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
What is electrophoresis?
A)a technique that separates amino acids on the basis of their polarity
B)a technique that separates amino acids on the basis of their solubility in water
C)a technique that separates amino acids on the basis of their pI values
D)a technique that separates amino acids on the basis of pKa of α-COOH values
E)a technique that separates amino acids on the basis of pKa of α-+NH3 values
A)a technique that separates amino acids on the basis of their polarity
B)a technique that separates amino acids on the basis of their solubility in water
C)a technique that separates amino acids on the basis of their pI values
D)a technique that separates amino acids on the basis of pKa of α-COOH values
E)a technique that separates amino acids on the basis of pKa of α-+NH3 values

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the isoelectric point for the following compound. 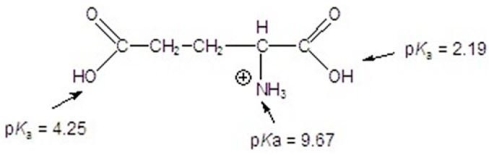
A)3)22
B)5)34
C)5)93
D)6)91
E)9)67

A)3)22
B)5)34
C)5)93
D)6)91
E)9)67

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the form in which glutamate exists at pH = 0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
What is thin-layer chromatography?
A)a technique that separates amino acids on the basis of their polarity
B)a technique that separates amino acids on the basis of their solubility in water
C)a technique that separates amino acids on the basis of their pI values
D)a technique that separates amino acids on the basis of pKa of α-COOH values
E)a technique that separates amino acids on the basis of pKa of α-+NH3 values
A)a technique that separates amino acids on the basis of their polarity
B)a technique that separates amino acids on the basis of their solubility in water
C)a technique that separates amino acids on the basis of their pI values
D)a technique that separates amino acids on the basis of pKa of α-COOH values
E)a technique that separates amino acids on the basis of pKa of α-+NH3 values

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following amino acids has the highest isoelectric point?
A)arginine
B)aspartic acid
C)glycine
D)proline
E)phenylalanine
A)arginine
B)aspartic acid
C)glycine
D)proline
E)phenylalanine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
Draw the predominant form of aspartate at physiological pH.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following best represents the structure of an amino acid in basic solution (pH = 11)?
A)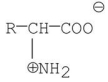
B)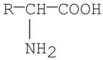
C)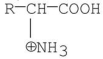
D)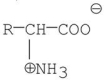
E)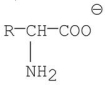
A)

B)

C)
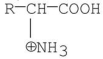
D)

E)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following best describes the side chain of arginine at a pH of 11?
A)hydrophobic
B)nonpolar
C)charged and polar
D)uncharged but polar
E)aprotic
A)hydrophobic
B)nonpolar
C)charged and polar
D)uncharged but polar
E)aprotic

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a zwitterion?
A)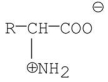
B)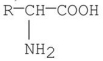
C)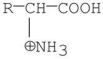
D)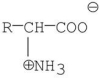
E)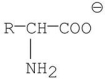
A)

B)
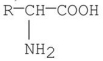
C)
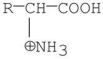
D)

E)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
Draw the predominant form of histidine at pH=0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the predominant form of proline at pH=12.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
Define what is meant by isoelectric point (pI)and give an example.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following amino acids has its isoelectric point at the lowest pH?
A)glutamic acid
B)lysine
C)valine
D)glycine
E)methionine
A)glutamic acid
B)lysine
C)valine
D)glycine
E)methionine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
What is the pI of glycine? The structure and pKa values are shown below. 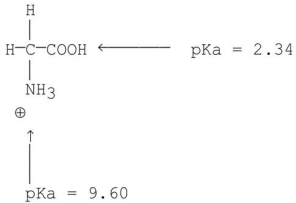
A)7)26
B)5)97
C)3)63
D)7)50
E)11.94

A)7)26
B)5)97
C)3)63
D)7)50
E)11.94

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
Give the pH at which histidine exists in the following form. 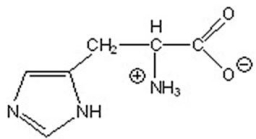
A)0
B)4
C)8
D)12
E)14

A)0
B)4
C)8
D)12
E)14

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
What amino acid can be obtained by the reductive amination of γ-ketoglutaric acid,HO2CCH2CH2COCO2H?
A)glycine
B)serine
C)lysine
D)aspartic acid
E)glutamic acid
A)glycine
B)serine
C)lysine
D)aspartic acid
E)glutamic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
What is the product obtained from the mild oxidation of the thiol shown below? 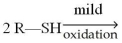
A)R-S-R
B)R-OH
C)R-S-S-R
D)R-OO-R
E)R-S-OH
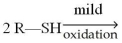
A)R-S-R
B)R-OH
C)R-S-S-R
D)R-OO-R
E)R-S-OH

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
Give the product for the following reaction. 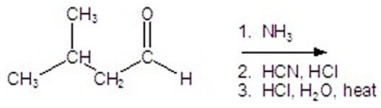
A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
What is an amino acid analyzer?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
What is the name of the amino acid produced when propanoic acid is subjected to the following sequence of reagents? 1.PBr3,Br2
2)H2O
3)NH3,Δ
A)alanine
B)aspartic acid
C)glutamic acid
D)valine
E)asparagine
2)H2O
3)NH3,Δ
A)alanine
B)aspartic acid
C)glutamic acid
D)valine
E)asparagine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
Give the product for the following reaction. 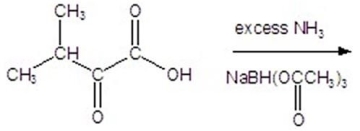
A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
Give the product for the following reaction. 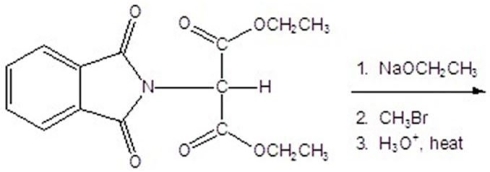
A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
Which labeled bond in the following molecule is known as the peptide bond? 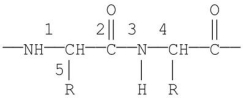
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
Rank the following amino acids in order of time of elution from a cation-exchange resin and a buffer pH of 4.0: aspartic acid,phenylalanine,arginine,and alanine.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Give the product for the following reaction. 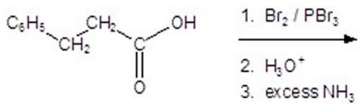


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
Give the product for the following reaction. 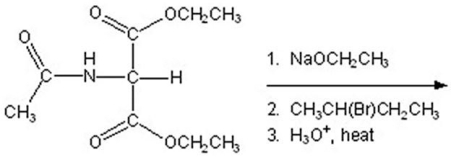
A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

A)alanine
B)leucine
C)valine
D)isoleucine
E)glycine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
Explain what is meant by kinetic resolution and give an example.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
Provide the major organic product(s)of the reaction below. 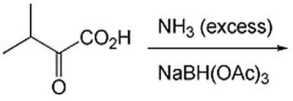


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
What is the major organic product of the following reaction? 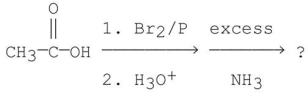
A)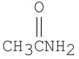
B)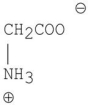
C)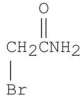
D)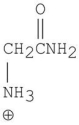
E)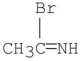

A)
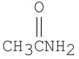
B)

C)

D)

E)
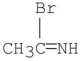

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
Provide the major organic product(s)of the reaction below. 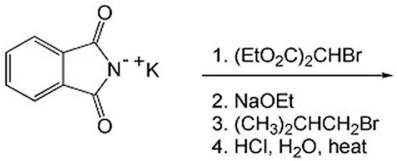


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
Provide the major organic product(s)of the reaction below. 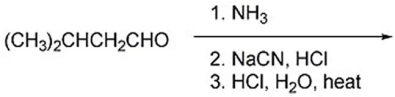


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
Rate the following amino acids in decreasing order of migration towards the cathode (negative electrode)when separated by electrophoresis in a solution of pH= 7.3.
I.lysine: pI= 9.87
II.alanine: pI= 6.02
III.aspartate: pI= 5.95
I.lysine: pI= 9.87
II.alanine: pI= 6.02
III.aspartate: pI= 5.95

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
You are given a mixture that contains glutamic acid (pI = 3.2),arginine (pI = 10.8),and valine (pI = 6.0),and you subject the mixture to electrophoresis.
a)Which amino acids migrate toward the cathode when the electrophoresis is carried out at a pH of 7.1?
b)Which amino acids migrate toward the anode when the electrophoresis is carried out at a pH of 7.1?
c)Which amino acid migrates farthest toward the anode at a pH of 7.1?
d)Since amino acids themselves are colorless,how is the separation of amino acids after electrophoresis detected?
a)Which amino acids migrate toward the cathode when the electrophoresis is carried out at a pH of 7.1?
b)Which amino acids migrate toward the anode when the electrophoresis is carried out at a pH of 7.1?
c)Which amino acid migrates farthest toward the anode at a pH of 7.1?
d)Since amino acids themselves are colorless,how is the separation of amino acids after electrophoresis detected?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is an acceptable name for the peptide sequence shown below? Glu-Glu-His-Val-Cys
A)1,2-diglutamylhistidylvalylcysteine
B)glutamylglutamylhistidylvalylcysteine
C)1-glutamyl-2-glutamylhistidylvalylcysteine
D)diglutamylhistidylvalylcysteine
E)biglutamylhistidylvalylcysteine
A)1,2-diglutamylhistidylvalylcysteine
B)glutamylglutamylhistidylvalylcysteine
C)1-glutamyl-2-glutamylhistidylvalylcysteine
D)diglutamylhistidylvalylcysteine
E)biglutamylhistidylvalylcysteine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
Describe how electrophoresis works.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
Provide the structure of the tripeptide val-gly-ser at pH 12.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the structure of the tripeptide glu-ser-ala at pH 12.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
Give a detailed,stepwise mechanism for the formation of the activated acyl derivative from the reaction of an amino acid with N,N'-dicyclohexylcarbodiimide.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
Provide the structure of Thr-Gln-Met at pH 7.0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the dipeptide Val-Tyr at pH 7.0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
In peptide synthesis,give the use of di-tert-butyl dicarbonate.
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
Explain the importance of the Merrifield method in peptide synthesis.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
In peptide synthesis,give the use of trifluoroacetic acid.
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
Provide the structure of the tripeptide val-gly-ser at pH 2.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following structures can polypeptides have?
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure
E)all of the above
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure
E)all of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
Draw the structure of the tetrapeptide Ser-Leu-Phe-Pro at pH 7.0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
In conventional peptide synthesis,the nitrogen of a given amino acid must be deactivated or blocked while the carboxyl group is activated.Which of the following reagents is used to protect the amino group of an amino acid?
A)di-tert-butyl dicarbonate
B)dicyclohexylcarbodiimide
C)ninhydrin
D)trifluoroacetic acid
E)phenylisothiocyanate
A)di-tert-butyl dicarbonate
B)dicyclohexylcarbodiimide
C)ninhydrin
D)trifluoroacetic acid
E)phenylisothiocyanate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
Which type of protein,globular or fibrous,tends to function primarily as structural parts of an organism?

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
Give the product for the following reaction. 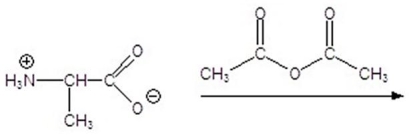
A)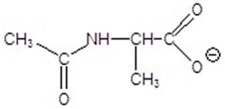
B)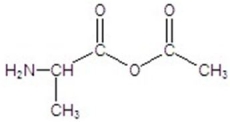
C)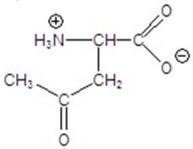
D)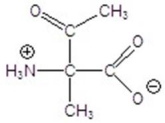
E)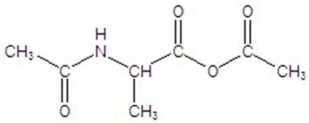

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
Provide the structure of Ala-Ser-Gly at biological pH.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
Provide the structure of the tripeptide glu-ser-ala at pH 2.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
In peptide synthesis,give the use of dicyclohexylcarbodiimide.
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid
A)activates the carboxyl group of an amino acid
B)protects the carboxyl group of an amino acid
C)protects the amino group of an amino acid
D)activates the amino group of an amino acid
E)removes the protecting group on the N-terminal amino acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
When a disulfide linkage is formed,the compound containing this new linkage has been
A)hydrolyzed.
B)dehydrated.
C)electrolyzed.
D)oxidized.
E)reduced.
A)hydrolyzed.
B)dehydrated.
C)electrolyzed.
D)oxidized.
E)reduced.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
Provide the structure of the tripeptide val-gly-ser at biological pH.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
Explain the chemistry behind giving a "permanent" to a head of hair.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck


