Deck 2: The Components of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/91
Play
Full screen (f)
Deck 2: The Components of Matter
1
Which of the following elements are the least reactive?
A)alkali metals
B)noble gases
C)halogens
D)alkaline earth metals
E)metalloids
A)alkali metals
B)noble gases
C)halogens
D)alkaline earth metals
E)metalloids
noble gases
2
Bromine is the only nonmetal that is a liquid at room temperature.Consider the isotope bromine-81, 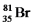
.Select the combination which lists the correct atomic number,neutron number,and mass number,respectively.
A)35,46,81
B)35,81,46
C)81,46,35
D)46,81,35
E)35,81,116
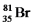
.Select the combination which lists the correct atomic number,neutron number,and mass number,respectively.
A)35,46,81
B)35,81,46
C)81,46,35
D)46,81,35
E)35,81,116
35,46,81
3
Who is credited with discovering the atomic nucleus?
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford
Rutherford
4
Who is credited with first measuring the charge of the electron?
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a metalloid?
A)carbon,C,Z = 6
B)sulfur,S,Z = 16
C)germanium,Ge,Z = 32
D)iridium,Z = 77
E)bromine,Br,Z = 35
A)carbon,C,Z = 6
B)sulfur,S,Z = 16
C)germanium,Ge,Z = 32
D)iridium,Z = 77
E)bromine,Br,Z = 35

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
6
Millikan's oil-drop experiment
A)established the charge on an electron.
B)showed that all oil drops carried the same charge.
C)provided support for the nuclear model of the atom.
D)suggested that some oil drops carried fractional numbers of electrons.
E)suggested the presence of a neutral particle in the atom.
A)established the charge on an electron.
B)showed that all oil drops carried the same charge.
C)provided support for the nuclear model of the atom.
D)suggested that some oil drops carried fractional numbers of electrons.
E)suggested the presence of a neutral particle in the atom.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
7
J.J.Thomson studied cathode ray particles (electrons)and was able to measure the mass/charge ratio.His results showed that
A)the mass/charge ratio varied as the cathode material was changed.
B)the charge was always a whole-number multiple of some minimum charge.
C)matter included particles much smaller than the atom.
D)atoms contained dense areas of positive charge.
E)atoms are largely empty space.
A)the mass/charge ratio varied as the cathode material was changed.
B)the charge was always a whole-number multiple of some minimum charge.
C)matter included particles much smaller than the atom.
D)atoms contained dense areas of positive charge.
E)atoms are largely empty space.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
8
Atoms X,Y,Z,and R have the following nuclear compositions: 
Which two are isotopes?
A)X & Y
B)X & R
C)Y & R
D)Z & R
E)X & Z

Which two are isotopes?
A)X & Y
B)X & R
C)Y & R
D)Z & R
E)X & Z

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
9
A column of the periodic table is called a
A)group
B)period
C)isotopic mixture
D)pillar
E)shell
A)group
B)period
C)isotopic mixture
D)pillar
E)shell

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
10
Compound 1 has a composition of 46.7 mass % of element A and 53.3 mass % of element B.A and B also form a second binary compound (compound 2).If the compositions of the two compounds are consistent with the law of multiple proportions,which of the following compositions could be that of compound 2?
A)23.4 mass % A 76.6 mass % B
B)30.4 mass % A 69.6 mass % B
C)33.3 mass % A 66.7 mass % B
D)53.3 mass % A 46.7 mass % B
E)73.3 mass % A 26.7 mass % B
A)23.4 mass % A 76.6 mass % B
B)30.4 mass % A 69.6 mass % B
C)33.3 mass % A 66.7 mass % B
D)53.3 mass % A 46.7 mass % B
E)73.3 mass % A 26.7 mass % B

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
11
A row of the periodic table is called a
A)group
B)period
C)isotopic mixture
D)family
E)subshell
A)group
B)period
C)isotopic mixture
D)family
E)subshell

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
12
Silicon,which makes up about 25% of Earth's crust by mass,is used widely in the modern electronics industry.It has three naturally occurring isotopes,28Si,29Si,and 30Si.Calculate the atomic mass of silicon.
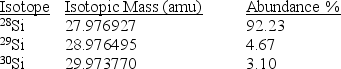
A)29.2252 amu
B)28.9757 amu
C)28.7260 amu
D)28.0855 amu
E)27.9801 amu
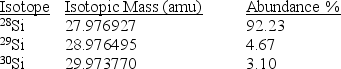
A)29.2252 amu
B)28.9757 amu
C)28.7260 amu
D)28.0855 amu
E)27.9801 amu

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
13
Who is credited with measuring the mass/charge ratio of the electron?
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford
A)Dalton
B)Gay-Lussac
C)Thomson
D)Millikan
E)Rutherford

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is a non-metal?
A)lithium,Li,Z = 3
B)bromine,Br,Z = 35
C)mercury,Hg,Z = 80
D)bismuth,Bi,Z = 83
E)sodium,Na,Z = 11
A)lithium,Li,Z = 3
B)bromine,Br,Z = 35
C)mercury,Hg,Z = 80
D)bismuth,Bi,Z = 83
E)sodium,Na,Z = 11

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following statements about atoms and subatomic particles is correct?
A)Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B)The proton and the neutron have identical masses.
C)The neutron's mass is equal to that of a proton plus an electron.
D)A neutral atom contains equal numbers of protons and electrons.
E)An atomic nucleus contains equal numbers of protons and neutrons.
A)Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B)The proton and the neutron have identical masses.
C)The neutron's mass is equal to that of a proton plus an electron.
D)A neutral atom contains equal numbers of protons and electrons.
E)An atomic nucleus contains equal numbers of protons and neutrons.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a metal?
A)nitrogen,N,Z = 7
B)phosphorus,P,Z = 15
C)arsenic,Z = 33
D)thallium,Tl,Z = 81
E)silicon,Si,Z = 14
A)nitrogen,N,Z = 7
B)phosphorus,P,Z = 15
C)arsenic,Z = 33
D)thallium,Tl,Z = 81
E)silicon,Si,Z = 14

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
17
Lithium forms compounds which are used in dry cells and storage batteries and in high-temperature lubricants.It has two naturally occurring isotopes,6Li (isotopic mass = 6.015121 amu)and 7Li (isotopic mass = 7.016003 amu).Lithium has an atomic mass of 6.9409 amu.What is the percent abundance of lithium-6?
A)92.50%
B)86.66%
C)46.16%
D)7.503%
E)6.080%
A)92.50%
B)86.66%
C)46.16%
D)7.503%
E)6.080%

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
18
Kaolinite,a clay mineral with the formula Al4Si4O10(OH)8,is used as a filler in slick-paper for magazines and as a raw material for ceramics.Analysis shows that 14.35 g of kaolinite contains 8.009 g of oxygen.Calculate the mass percent of oxygen in kaolinite.
A)1.792 mass %
B)24.80 mass %
C)30.81 mass %
D)34.12 mass %
E)55.81 mass %
A)1.792 mass %
B)24.80 mass %
C)30.81 mass %
D)34.12 mass %
E)55.81 mass %

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
19
In a Millikan oil-drop experiment,the charges on several different oil drops were as follows: -5.92;-4.44;-2.96;-8.88.The units are arbitrary.What is the likely value of the electronic charge in these arbitrary units?
A)-1.11
B)-1.48
C)-2.22
D)-2.96
E)-5.55
A)-1.11
B)-1.48
C)-2.22
D)-2.96
E)-5.55

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
20
Rutherford bombarded gold foil with alpha ( )particles and found that a small percentage of the particles were deflected.Which of the following was not accounted for by the model he proposed for the structure of atoms?
A)the small size of the nucleus
B)the charge on the nucleus
C)the total mass of the atom
D)the existence of protons
E)the presence of electrons outside the nucleus
A)the small size of the nucleus
B)the charge on the nucleus
C)the total mass of the atom
D)the existence of protons
E)the presence of electrons outside the nucleus

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
21
The substance,CaSe,is used in materials which are electron emitters.What is its name?
A)calcium monoselenide
B)calcium(II)selenide
C)calcium selenide
D)calcium(I)selenide
E)calcium(II)selenium
A)calcium monoselenide
B)calcium(II)selenide
C)calcium selenide
D)calcium(I)selenide
E)calcium(II)selenium

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following combinations of names and formulas of ions is incorrect?
A)NH4+ ammonium
B)S2- sulfide
C)CN- cyanide
D)S2O32- thiosulfate
E)ClO3- perchlorate
A)NH4+ ammonium
B)S2- sulfide
C)CN- cyanide
D)S2O32- thiosulfate
E)ClO3- perchlorate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
23
Barium fluoride is used in embalming and in glass manufacturing.Which of the following gives the formula and bonding for barium fluoride?
A)BaF2,ionic compound
B)BaF2,covalent compound
C)BaF,ionic compound
D)BaF,covalent compound
E)Ba2F,ionic compound
A)BaF2,ionic compound
B)BaF2,covalent compound
C)BaF,ionic compound
D)BaF,covalent compound
E)Ba2F,ionic compound

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
24
The compound,BaO,absorbs water and carbon dioxide readily and is used to dry gases and organic solvents.What is its name?
A)barium oxide
B)barium(II)oxide
C)barium monoxide
D)baric oxide
E)barium peroxide
A)barium oxide
B)barium(II)oxide
C)barium monoxide
D)baric oxide
E)barium peroxide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
25
What is the name of Na2O?
A)disodium monoxide
B)sodium monoxide
C)sodium dioxide
D)sodium(I)oxide
E)sodium oxide
A)disodium monoxide
B)sodium monoxide
C)sodium dioxide
D)sodium(I)oxide
E)sodium oxide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
26
Sodium oxide combines violently with water.Which of the following gives the formula and the bonding for sodium oxide?
A)NaO,ionic compound
B)NaO,covalent compound
C)Na2O,ionic compound
D)Na2O,covalent compound
E)Na2O2,ionic compound
A)NaO,ionic compound
B)NaO,covalent compound
C)Na2O,ionic compound
D)Na2O,covalent compound
E)Na2O2,ionic compound

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
27
The colorless substance,MgF2,is used in the ceramics and glass industry.What is its name?
A)magnesium difluoride
B)magnesium fluoride
C)magnesium(II)fluoride
D)monomagnesium difluoride
E)none of these choices is correct,since they are all misspelled
A)magnesium difluoride
B)magnesium fluoride
C)magnesium(II)fluoride
D)monomagnesium difluoride
E)none of these choices is correct,since they are all misspelled

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds is covalent?
A)CaCl2
B)MgO
C)Al2O3
D)Cs2S
E)PCl3
A)CaCl2
B)MgO
C)Al2O3
D)Cs2S
E)PCl3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following compounds is ionic?
A)PF3
B)CS2
C)HCl
D)SO2
E)MgCl2
A)PF3
B)CS2
C)HCl
D)SO2
E)MgCl2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
30
A red glaze on porcelain can be produced by using MnSO4.What is its name?
A)manganese disulfate
B)manganese(II)sulfate
C)manganese(IV)sulfate
D)manganese sulfate
E)manganese(I)sulfate
A)manganese disulfate
B)manganese(II)sulfate
C)manganese(IV)sulfate
D)manganese sulfate
E)manganese(I)sulfate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following combinations of names and formulas of ions is incorrect?
A)Ba2+ barium
B)S2- sulfate
C)CN- cyanide
D)ClO4- perchlorate
E)HCO3- bicarbonate
A)Ba2+ barium
B)S2- sulfate
C)CN- cyanide
D)ClO4- perchlorate
E)HCO3- bicarbonate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following combinations of names and formulas of ions is incorrect?
A)O2- oxide
B)Cd2+ cadmium
C)ClO3- chlorate
D)HCO3- hydrogen carbonate
E)NO2- nitrate
A)O2- oxide
B)Cd2+ cadmium
C)ClO3- chlorate
D)HCO3- hydrogen carbonate
E)NO2- nitrate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following ions occurs commonly?
A)N3+
B)S6+
C)O2-
D)Ca+
E)Cl+
A)N3+
B)S6+
C)O2-
D)Ca+
E)Cl+

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
34
The compound, (NH4)2S,can be used in analysis for trace amounts of metals present in a sample.What is its name?
A)ammonium sulfide
B)diammonium sulfide
C)ammonium sulfite
D)ammonia(I)sulfite
E)ammonium(I)sulfide
A)ammonium sulfide
B)diammonium sulfide
C)ammonium sulfite
D)ammonia(I)sulfite
E)ammonium(I)sulfide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
35
The substance,CoCl2,is useful as a humidity indicator because it changes from pale blue to pink as it gains water from moist air.What is its name?
A)cobalt dichloride
B)cobalt(II)chloride
C)cobalt chloride
D)cobaltic chloride
E)copper(II)chloride
A)cobalt dichloride
B)cobalt(II)chloride
C)cobalt chloride
D)cobaltic chloride
E)copper(II)chloride

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the empirical formula for hexane,C6H14?
A)C12H28
B)C6H14
C)C3H7
D)CH2.3
E)C0.43H
A)C12H28
B)C6H14
C)C3H7
D)CH2.3
E)C0.43H

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
37
The compound,NaH2PO4,is present in many baking powders.What is its name?
A)sodium biphosphate
B)sodium hydrogen phosphate
C)sodium dihydrogen phosphate
D)sodium hydrophosphate
E)sodium dihydride phosphate
A)sodium biphosphate
B)sodium hydrogen phosphate
C)sodium dihydrogen phosphate
D)sodium hydrophosphate
E)sodium dihydride phosphate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following ions occurs commonly?
A)P3+
B)Br7+
C)O6+
D)Ca2+
E)K-
A)P3+
B)Br7+
C)O6+
D)Ca2+
E)K-

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
39
The substance,KClO3,is a strong oxidizer used in explosives,fireworks,and matches.What is its name?
A)potassium chlorite
B)potassium chloride
C)potassium(I)chlorite
D)potassium(I)chlorate
E)potassium chlorate
A)potassium chlorite
B)potassium chloride
C)potassium(I)chlorite
D)potassium(I)chlorate
E)potassium chlorate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
40
Which one of the following combinations of names and formulas of ions is incorrect?
A)O2- oxide
B)Al3+ aluminum
C)NO3- nitrate
D)PO43- phosphate
E)CrO42- chromate
A)O2- oxide
B)Al3+ aluminum
C)NO3- nitrate
D)PO43- phosphate
E)CrO42- chromate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
41
Which one of the following formulas of ionic compounds is the least likely to be correct?
A)NH4Cl
B)Ba(OH)2
C)Na2SO4
D)Ca2NO3
E)Cu(CN)2
A)NH4Cl
B)Ba(OH)2
C)Na2SO4
D)Ca2NO3
E)Cu(CN)2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
42
What is the formula for lithium nitrite?
A)LiNO2
B)Li2NO2
C)LiNO3
D)Li2NO3
E)LiNO4
A)LiNO2
B)Li2NO2
C)LiNO3
D)Li2NO3
E)LiNO4

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula for magnesium sulfide?
A)MgS
B)MgS2
C)Mg2S
D)Mg2S3
E)MgSO4
A)MgS
B)MgS2
C)Mg2S
D)Mg2S3
E)MgSO4

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
44
Silver chloride is used in photographic emulsions.What is its formula?
A)Ag2Cl3
B)Ag2Cl
C)AgCl3
D)AgCl2
E)AgCl
A)Ag2Cl3
B)Ag2Cl
C)AgCl3
D)AgCl2
E)AgCl

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
45
Ferric oxide is used as a pigment in metal polishing.Which of the following is its formula?
A)FeO
B)Fe2O
C)FeO3
D)Fe2O5
E)Fe2O3
A)FeO
B)Fe2O
C)FeO3
D)Fe2O5
E)Fe2O3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of PCl3?
A)phosphorus chloride
B)phosphoric chloride
C)phosphorus trichlorate
D)trichlorophosphide
E)phosphorus trichloride
A)phosphorus chloride
B)phosphoric chloride
C)phosphorus trichlorate
D)trichlorophosphide
E)phosphorus trichloride

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
47
The compound,P4S10,is used in the manufacture of safety matches.What is its name?
A)phosphorus sulfide
B)phosphoric sulfide
C)phosphorus decasulfide
D)tetraphosphorus decasulfide
A)phosphorus sulfide
B)phosphoric sulfide
C)phosphorus decasulfide
D)tetraphosphorus decasulfide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the acid formed when H2S gas is dissolved in water?
A)sulfuric acid
B)sulfurous acid
C)hydrosulfuric acid
D)hydrosulfurous acid
E)sulfidic acid
A)sulfuric acid
B)sulfurous acid
C)hydrosulfuric acid
D)hydrosulfurous acid
E)sulfidic acid

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
49
Sodium peroxide is an oxidizer used to bleach animal and vegetable fibers.What is its formula?
A)NaO
B)NaO2
C)Na2O2
D)Na2O
E)NaH2O2
A)NaO
B)NaO2
C)Na2O2
D)Na2O
E)NaH2O2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
50
What is the name of the acid formed when HBr gas is dissolved in water?
A)bromic acid
B)bromous acid
C)hydrobromic acid
D)hydrobromous acid
E)hydrobromidic acid
A)bromic acid
B)bromous acid
C)hydrobromic acid
D)hydrobromous acid
E)hydrobromidic acid

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
51
Which one of the following combinations of names and formulas is incorrect?
A)H3PO4 phosphoric acid
B)HNO3 nitric acid
C)NaHCO3 sodium carbonate
D)H2CO3 carbonic acid
E)KOH potassium hydroxide
A)H3PO4 phosphoric acid
B)HNO3 nitric acid
C)NaHCO3 sodium carbonate
D)H2CO3 carbonic acid
E)KOH potassium hydroxide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
52
Barium sulfate is used in manufacturing photographic paper.What is its formula?
A)BaSO4
B)Ba(SO4)2
C)Ba2SO4
D)Ba2(SO4)3
E)BaSO3
A)BaSO4
B)Ba(SO4)2
C)Ba2SO4
D)Ba2(SO4)3
E)BaSO3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
53
What is the name of the acid formed when HClO4 liquid is dissolved in water?
A)hydrochloric acid
B)perchloric acid
C)chloric acid
D)chlorous acid
E)hydrochlorate acid
A)hydrochloric acid
B)perchloric acid
C)chloric acid
D)chlorous acid
E)hydrochlorate acid

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
54
Which one of the following formulas of ionic compounds is the least likely to be correct?
A)CaCl2
B)NaSO4
C)MgCO3
D)KF
E)Cu(NO3)2
A)CaCl2
B)NaSO4
C)MgCO3
D)KF
E)Cu(NO3)2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
55
Zinc acetate is used in preserving wood and in manufacturing glazes for porcelain.What is its formula?
A)ZnAc2
B)ZnCH3COO
C)Zn(CH3COO)2
D)Zn2CH3COO
E)ZnCH3COCH3
A)ZnAc2
B)ZnCH3COO
C)Zn(CH3COO)2
D)Zn2CH3COO
E)ZnCH3COCH3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
56
Potassium permanganate is a strong oxidizer that reacts explosively with easily oxidized materials.What is its formula?
A)KMnO3
B)KMnO4
C)K2MnO4
D)K(MnO4)2
E)K2Mn2O7
A)KMnO3
B)KMnO4
C)K2MnO4
D)K(MnO4)2
E)K2Mn2O7

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
57
Calcium hydroxide is used in mortar,plaster and cement.What is its formula?
A)CaOH
B)CaOH2
C)Ca2OH
D)Ca(OH)2
E)CaHO2
A)CaOH
B)CaOH2
C)Ca2OH
D)Ca(OH)2
E)CaHO2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
58
What is the formula for lead (II)oxide?
A)PbO
B)PbO2
C)Pb2O
D)PbO4
E)Pb2O3
A)PbO
B)PbO2
C)Pb2O
D)PbO4
E)Pb2O3

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
59
Iron (III)chloride hexahydrate is used as a coagulant for sewage and industrial wastes.What is its formula?
A)Fe(Cl·6H2O)3
B)Fe3Cl·6H2O
C)FeCl3(H2O)6
D)Fe3Cl(H2O)6
E)FeCl3·6H2O
A)Fe(Cl·6H2O)3
B)Fe3Cl·6H2O
C)FeCl3(H2O)6
D)Fe3Cl(H2O)6
E)FeCl3·6H2O

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
60
What is the name of the acid formed when HCN gas is dissolved in water?
A)cyanic acid
B)hydrocyanic acid
C)cyanous acid
D)hydrocyanous acid
E)hydrogen cyanide
A)cyanic acid
B)hydrocyanic acid
C)cyanous acid
D)hydrocyanous acid
E)hydrogen cyanide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
61
Name the three important "laws" that were accounted for by Dalton's atomic theory.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
62
State the two important experimental results (and the names of the responsible scientists)which enabled the mass of the electron to be determined.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
63
Ammonium sulfate, (NH4)2SO4,is a fertilizer widely used as a source of nitrogen.Calculate its molecular mass.
A)63.07 amu
B)114.l0 amu
C)118.13 amu
D)128.11 amu
E)132.13 amu
A)63.07 amu
B)114.l0 amu
C)118.13 amu
D)128.11 amu
E)132.13 amu

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the molecular mass of iron (III)bromide hexahydrate,a substance used as a catalyst in organic reactions.
A)403.65 amu
B)355.54 amu
C)317.61 amu
D)313.57 amu
E)295.56 amu
A)403.65 amu
B)355.54 amu
C)317.61 amu
D)313.57 amu
E)295.56 amu

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
65
Fill in the blank spaces and write out all the symbols in the left hand column in full,in the form 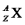
(i.e. ,include the appropriate values of Z and A as well as the correct symbol X).

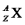
(i.e. ,include the appropriate values of Z and A as well as the correct symbol X).


Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
66
What is the name of P4Se3?
A)phosphorus selenide
B)phosphorus triselenide
C)tetraphosphorus selenide
D)phosphoric selenide
E)tetraphosphorus triselenide
A)phosphorus selenide
B)phosphorus triselenide
C)tetraphosphorus selenide
D)phosphoric selenide
E)tetraphosphorus triselenide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
67
The following charges on individual oil droplets were obtained during an experiment similar to Millikan's.Use them to determine a charge for the electron in coulombs (C),showing all your working.
Charges (C): -3.184 10-19;-4.776 10-19;-7.960 10-19
Charges (C): -3.184 10-19;-4.776 10-19;-7.960 10-19

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
68
Iodine pentafluoride reacts slowly with glass and violently with water.Determine its molecular mass.
A)653.52 amu
B)259.89 amu
C)221.90 amu
D)202.90 amu
E)145.90 amu
A)653.52 amu
B)259.89 amu
C)221.90 amu
D)202.90 amu
E)145.90 amu

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of IF7?
A)iodine fluoride
B)iodic fluoride
C)iodine heptafluoride
D)heptafluoroiodide
E)heptafluorine iodide
A)iodine fluoride
B)iodic fluoride
C)iodine heptafluoride
D)heptafluoroiodide
E)heptafluorine iodide

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
70
Diiodine pentaoxide is used as an oxidizing agent that converts carbon monoxide to carbon dioxide.What is its chemical formula?
A)I2O5
B)IO5
C)2IO5
D)I5O2
E)(IO5)2
A)I2O5
B)IO5
C)2IO5
D)I5O2
E)(IO5)2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
71
For each of the following names,write down the corresponding formula,including charge where appropriate (atomic numbers and mass numbers are not required):
a.zinc ion
b.nitrite ion
c.carbonic acid
d.cyanide ion
a.zinc ion
b.nitrite ion
c.carbonic acid
d.cyanide ion

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
72
a.Give the names of the following ions:
(i)NH4+
(ii)SO32-
b.Write down the formulas of the following ions:
(i)aluminum
(ii)carbonate
(i)NH4+
(ii)SO32-
b.Write down the formulas of the following ions:
(i)aluminum
(ii)carbonate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
73
What is the name of BBr3?
A)boron bromide
B)boric bromide
C)boron tribromide
D)tribromoboride
E)bromine triboride
A)boron bromide
B)boric bromide
C)boron tribromide
D)tribromoboride
E)bromine triboride

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
74
Chlorine dioxide is a strong oxidizer that is used for bleaching flour and textiles and for purification of water.What is its formula?
A)(ClO)2
B)Cl2O
C)Cl2O2
D)Cl2O4
E)ClO2
A)(ClO)2
B)Cl2O
C)Cl2O2
D)Cl2O4
E)ClO2

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
75
Dalton's atomic theory has required some modifications in the light of subsequent discoveries.For any two appropriate postulates of Dalton's atomic theory
a.state the postulate in its original form
b.In one sentence,describe why the postulate has needed modification.
a.state the postulate in its original form
b.In one sentence,describe why the postulate has needed modification.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
76
Give the common name of the group in the periodic table to which each of the following elements belongs:
a.Rb
b.Br
c.Ba
d.Ar
a.Rb
b.Br
c.Ba
d.Ar

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
77
For each of the following elements,indicate whether it is a metal,a non-metal or a metalloid:
a.S
b.Ge
c.Hg
d.H
e.I
f.Si
a.S
b.Ge
c.Hg
d.H
e.I
f.Si

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
78
Sodium chromate is used to protect iron from corrosion and rusting.Determine its molecular mass.
A)261.97 amu
B)238.98 amu
C)161.97 amu
D)138.98 amu
E)74.99 amu
A)261.97 amu
B)238.98 amu
C)161.97 amu
D)138.98 amu
E)74.99 amu

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
79
Tetrasulfur dinitride decomposes explosively when heated.What is its formula?
A)S2N4
B)S4N2
C)4SN2
D)S4N
E)S2N
A)S2N4
B)S4N2
C)4SN2
D)S4N
E)S2N

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
80
a.Give the names of the following ions:
(i)O22-
(ii)SO42-
b.Write down the formulas of the following ions:
(i)ammonium
(ii)nitrate
(i)O22-
(ii)SO42-
b.Write down the formulas of the following ions:
(i)ammonium
(ii)nitrate

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck


