Deck 5: Gases and the Kinetic-Molecular Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 5: Gases and the Kinetic-Molecular Theory
1
Which of the lines on the figure below is the best representation of the relationship between the volume and the number of moles of a gas,measured at constant temperature and pressure? 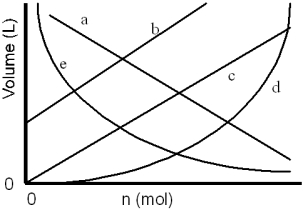
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e
c
2
The pressure of hydrogen sulfide gas in a container is 35,650 Pa.What is this pressure in torr?
A)46.91 torr
B)267.4 torr
C)351.8 torr
D)3612 torr
E)27,090 torr
A)46.91 torr
B)267.4 torr
C)351.8 torr
D)3612 torr
E)27,090 torr
267.4 torr
3
"The volume of an ideal gas is directly proportional to its absolute temperature at constant pressure and number of moles" is a statement of ________________ Law.
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Dalton's
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Dalton's
Charles'
4
The air pressure in a volleyball is 75 psi.What is this pressure in torr?
A)520 torr
B)562 torr
C)3900 torr
D)7600 torr
E)75,000 torr
A)520 torr
B)562 torr
C)3900 torr
D)7600 torr
E)75,000 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
A sample of nitrogen gas at 298 K and 745 torr has a volume of 37.42 L.What volume will it occupy if the pressure is increased to 894 torr at constant temperature?
A)22.3 L
B)31.2 L
C)44.9 L
D)112 L
E)380 L
A)22.3 L
B)31.2 L
C)44.9 L
D)112 L
E)380 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
Hydrogen gas exerts a pressure of 466 torr in a container.What is this pressure in atmospheres?
A)0.217 atm
B)0.466 atm
C)0.613 atm
D)1.63 atm
E)4.60 atm
A)0.217 atm
B)0.466 atm
C)0.613 atm
D)1.63 atm
E)4.60 atm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
Mineral oil can be used in place of mercury in certain pressure-measuring instruments when small pressure changes are to be measured.What is the pressure of an oxygen sample in mm of mineral oil if its pressure is 28.5 mm Hg? (d of mineral oil = 0.88 g/mL;d of Hg = 13.5 g/mL)
A)1.9 mm mineral oil
B)15 mm mineral oil
C)32 mm mineral oil
D)380 mm mineral oil
E)440 mm mineral oil
A)1.9 mm mineral oil
B)15 mm mineral oil
C)32 mm mineral oil
D)380 mm mineral oil
E)440 mm mineral oil

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
"The total pressure in a mixture of unreacting gases is equal to the sum of the partial pressures of the individual gases" is a statement of __________________ Law.
A)Charles'
B)Graham's
C)Boyle's
D)Avogadro's
E)Dalton's
A)Charles'
B)Graham's
C)Boyle's
D)Avogadro's
E)Dalton's

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
"The volume of an ideal gas is directly proportional to the number of moles of the gas at constant temperature and pressure" is a statement of _____________ Law.
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Dalton's
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Dalton's

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
A sample container of carbon monoxide occupies a volume of 435 mL at a pressure of 785 torr and a temperature of 298 K.What would its temperature be if the volume were changed to 265 mL at a pressure of 785 torr?
A)182 K
B)298 K
C)387 K
D)489 K
E)538 K
A)182 K
B)298 K
C)387 K
D)489 K
E)538 K

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
A sample of oxygen gas has its absolute temperature halved while the pressure of the gas remained constant.If the initial volume is 400 mL,what is the final volume?
A)20 mL
B)133 mL
C)200 mL
D)400 mL
E)800 mL
A)20 mL
B)133 mL
C)200 mL
D)400 mL
E)800 mL

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
"The rate of effusion of a gas is inversely proportional to the square root of its molar mass" is a statement of _________________ Law.
A)Charles'
B)Graham's
C)Dalton's
D)Avogadro's
E)Boyle's
A)Charles'
B)Graham's
C)Dalton's
D)Avogadro's
E)Boyle's

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its pressure,other factors remaining constant? 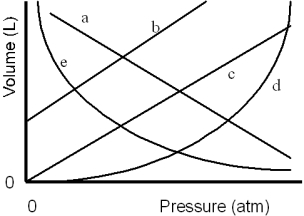
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
The pressure of sulfur dioxide in a container is 159 kPa.What is this pressure in atmospheres?
A)0.209 atm
B)0.637 atm
C)1.57 atm
D)21.2 atm
E)15,900 atm
A)0.209 atm
B)0.637 atm
C)1.57 atm
D)21.2 atm
E)15,900 atm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its Celsius temperature,other factors remaining constant? 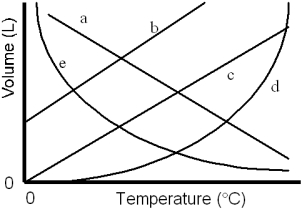
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its absolute temperature,other factors remaining constant? 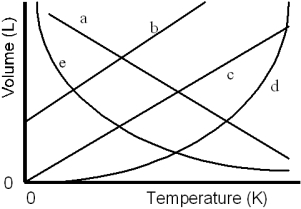
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
A sample of the inert gas krypton has its pressure tripled while its temperature remained constant.If the original volume is 12 L,what is the final volume?
A)4.0 L
B)6.0 L
C)9 L
D)36 L
E)48 L
A)4.0 L
B)6.0 L
C)9 L
D)36 L
E)48 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L.What will the gas pressure be if the volume is increased to 321 L at 125°C?
A)212 torr
B)289 torr
C)356 torr
D)441 torr
E)359 torr
A)212 torr
B)289 torr
C)356 torr
D)441 torr
E)359 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
A sample of an ideal gas has its volume doubled while its temperature remains constant.If the original pressure was 100 torr,what is the new pressure?
A)10 torr
B)50 torr
C)100 torr
D)200 torr
E)1000 torr
A)10 torr
B)50 torr
C)100 torr
D)200 torr
E)1000 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
"The pressure of an ideal gas is inversely proportional to its volume at constant temperature and number of moles" is a statement of __________________ Law.
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Gay-Lussac's
A)Charles'
B)Boyle's
C)Amontons'
D)Avogadro's
E)Gay-Lussac's

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
A 500-mL sample of argon at 800 torr has its absolute temperature quadrupled.If the volume remains unchanged,what is the new pressure?
A)200 torr
B)400 torr
C)800 torr
D)2400 torr
E)3200 torr
A)200 torr
B)400 torr
C)800 torr
D)2400 torr
E)3200 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
A sample of methane gas,CH4(g),occupies a volume of 60.3 L at a pressure of 469 torr and a temperature of 29.3°C.What would be its temperature at a pressure of 243 torr and volume of 60.3 L?
A)-116.5°C
B)15.2°C
C)15.5°C
D)57.7°C
E)310.6°C
A)-116.5°C
B)15.2°C
C)15.5°C
D)57.7°C
E)310.6°C

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
A sample of ammonia gas at 65.5°C and 524 torr has a volume of 15.31 L.What is its volume when the temperature is -15.8°C and its pressure is 524 torr?
A)3.69 L
B)11.6 L
C)20.2 L
D)63.5 L
E)It is not possible,since the volume would have to be negative.
A)3.69 L
B)11.6 L
C)20.2 L
D)63.5 L
E)It is not possible,since the volume would have to be negative.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
Ima Chemist found the density of Freon-11 (CFCl3)to be 5.58 g/L under her experimental conditions.Her measurements showed that the density of an unknown gas was 4.38 g/L under the same conditions.What is the molar mass of the unknown?
A)96.7 g/mol
B)108 g/mol
C)127 g/mol
D)165 g/mol
E)175 g/mol
A)96.7 g/mol
B)108 g/mol
C)127 g/mol
D)165 g/mol
E)175 g/mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
A 0.850-mole sample of nitrous oxide,a gas used as an anesthetic by dentists,has a volume of 20.46 L at 123°C and 1.35 atm.What would be its volume at 468°C and 1.35 atm?
A)5.38 L
B)10.9 L
C)19.0 L
D)38.3 L
E)77.9 L
A)5.38 L
B)10.9 L
C)19.0 L
D)38.3 L
E)77.9 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
A 750-mL sample of hydrogen exerts a pressure of 822 torr at 325 K.What pressure does it exert if the temperature is raised to 475 K at constant volume?
A)188 torr
B)562 torr
C)1.11 103 torr
D)1.20 103 torr
E)1.90 103 torr
A)188 torr
B)562 torr
C)1.11 103 torr
D)1.20 103 torr
E)1.90 103 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the pressure of a helium sample at -207.3°C and 768 mL if it exerts a pressure of 175 kPa at 25.0°C and 925 mL.
A)32.1 kPa
B)46.6 kPa
C)657 kPa
D)953 kPa
E)It is not possible,since the pressure would have to be negative.
A)32.1 kPa
B)46.6 kPa
C)657 kPa
D)953 kPa
E)It is not possible,since the pressure would have to be negative.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr.What is its volume at STP?
A)22.4 L
B)31.1 L
C)34.3 L
D)35.2 L
E)47.7 L
A)22.4 L
B)31.1 L
C)34.3 L
D)35.2 L
E)47.7 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm.What is the molar mass of the gas?
A)7.76 g/mol
B)66.1 g/mol
C)74.0 g/mol
D)81.4 g/mol
E)144 g/mol
A)7.76 g/mol
B)66.1 g/mol
C)74.0 g/mol
D)81.4 g/mol
E)144 g/mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of nitrogen gas is confined to a 14.0 L container at 375 torr and 37.0°C.How many moles of nitrogen are in the container?
A)0.271 mol
B)2.27 mol
C)3.69 mol
D)206 mol
E)227 mol
A)0.271 mol
B)2.27 mol
C)3.69 mol
D)206 mol
E)227 mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
Dr.I.M.A.Brightguy adds 0.1727 g of an unknown gas to a 125-mL flask.If Dr.B finds the pressure to be 736 torr at 20.0°C,is the gas likely to be methane,CH4,nitrogen,N2,oxygen,O2,neon,Ne,or argon,Ar?
A)CH4
B)N2
C)Ne
D)Ar
E)O2
A)CH4
B)N2
C)Ne
D)Ar
E)O2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies 25.8 L at 75.0°C and 41.1 kPa.
A)95.0°C
B)85.1°C
C)77.2°C
D)72.9°C
E)65.2°C
A)95.0°C
B)85.1°C
C)77.2°C
D)72.9°C
E)65.2°C

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
A 250.0-mL sample of ammonia,NH3(g),exerts a pressure of 833 torr at 42.4°C.What mass of ammonia is in the container?
A)0.0787 g
B)0.180 g
C)8.04 g
D)17.0 g
E)59.8 g
A)0.0787 g
B)0.180 g
C)8.04 g
D)17.0 g
E)59.8 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
Nitrogen dioxide is a red-brown gas that is responsible for the color of photochemical smog.A sample of nitrogen dioxide has a volume of 28.6 L at 45.3°C and 89.9 kPa.What is its volume at STP?
A)21.8 L
B)27.6 L
C)29.6 L
D)37.6 L
E)153 L
A)21.8 L
B)27.6 L
C)29.6 L
D)37.6 L
E)153 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
What are the conditions of STP?
A)0 K and l atm
B)273.15 K and 760 torr
C)0°C and 760 atm
D)273.15°C and 760 torr
E)None of these choices is correct.
A)0 K and l atm
B)273.15 K and 760 torr
C)0°C and 760 atm
D)273.15°C and 760 torr
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
What is the density of carbon dioxide gas at -25.2°C and 98.0 kPa?
A)0.232 g/L
B)0.279 g/L
C)0.994 g/L
D)1.74 g/L
E)2.09 g/L
A)0.232 g/L
B)0.279 g/L
C)0.994 g/L
D)1.74 g/L
E)2.09 g/L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
What is the pressure in a 7.50-L flask if 0.15 mol of carbon dioxide is added to 0.33 mol of oxygen? The temperature of the mixture is 48.0°C.
A)0.252 atm
B)0.592 atm
C)1.69 atm
D)3.96 atm
E)4.80 atm
A)0.252 atm
B)0.592 atm
C)1.69 atm
D)3.96 atm
E)4.80 atm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of propane,a component of LP gas,has a volume of 35.3 L at 315 K and 922 torr.What is its volume at STP?
A)25.2 L
B)30.6 L
C)33.6 L
D)37.1 L
E)49.2 L
A)25.2 L
B)30.6 L
C)33.6 L
D)37.1 L
E)49.2 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
A compressed gas cylinder containing 1.50 mol methane has a volume of 3.30 L.What pressure does the methane exert on the walls of the cylinder if its temperature is 25°C?
A)9.00 10-2 atm
B)0.933 atm
C)1.11 atm
D)1.70 atm
E)11.1 atm
A)9.00 10-2 atm
B)0.933 atm
C)1.11 atm
D)1.70 atm
E)11.1 atm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
Assuming ideal behavior,what is the density of argon gas at STP,in g/L?
A)0.0176 g/L
B)0.0250 g/L
C)0.0561 g/L
D)1.78 g/L
E)181.g/L
A)0.0176 g/L
B)0.0250 g/L
C)0.0561 g/L
D)1.78 g/L
E)181.g/L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following gases effuses most rapidly?
A)nitrogen
B)oxygen
C)hydrogen chloride
D)ammonia
E)carbon monoxide
A)nitrogen
B)oxygen
C)hydrogen chloride
D)ammonia
E)carbon monoxide

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
Freon-12,CF2Cl2,which has been widely used in air conditioning systems,is considered a threat to the ozone layer in the stratosphere.Calculate the root-mean-square velocity of Freon-12 molecules in the lower stratosphere where the temperature is -65°C.
A)20 m/s
B)120 m/s
C)210 m/s
D)260 m/s
E)4.4 104 m/s
A)20 m/s
B)120 m/s
C)210 m/s
D)260 m/s
E)4.4 104 m/s

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the root-mean-square speed of methane,CH4 (g),at 78°C.
A)23 m/s
B)350 m/s
C)550 m/s
D)667 m/s
E)740 m/s
A)23 m/s
B)350 m/s
C)550 m/s
D)667 m/s
E)740 m/s

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
A gas mixture,with a total pressure of 300.torr,consists of equal masses of Ne (atomic weight 20. )and Ar (atomic weight 40. ).What is the partial pressure of Ar,in torr?
A)75 torr
B)100.torr
C)150.torr
D)200.torr
E)None of these choices is correct.
A)75 torr
B)100.torr
C)150.torr
D)200.torr
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
An unknown liquid is vaporized in a 273-mL flask by immersion in a water bath at 99°C.The barometric pressure is 753 torr.If the mass of the substance's vapor retained in the flask is 1.362 g,what is its molar mass?
A)20.4 g/mol
B)40.9 g/mol
C)112 g/mol
D)154 g/mol
E)184 g/mol
A)20.4 g/mol
B)40.9 g/mol
C)112 g/mol
D)154 g/mol
E)184 g/mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
If 0.750 L of argon at 1.50 atm and 177°C and 0.235 L of sulfur dioxide at 95.0 kPa and 63.0°C are added to a 1.00-L flask and the flask's temperature is adjusted to 25.0°C,what is the resulting pressure in the flask?
A)0.0851 atm
B)0.244 atm
C)0.946 atm
D)1.74 atm
E)1.86 atm
A)0.0851 atm
B)0.244 atm
C)0.946 atm
D)1.74 atm
E)1.86 atm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
Small quantities of hydrogen can be prepared by the addition of hydrochloric acid to zinc.A sample of 195 mL of hydrogen was collected over water at 25°C and 753 torr.What mass of hydrogen was collected? (Pwater = 24 torr at 25°C)
A)0.00765 g
B)0.0154 g
C)0.0159 g
D)0.0164 g
E)0.159 g
A)0.00765 g
B)0.0154 g
C)0.0159 g
D)0.0164 g
E)0.159 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
A gas mixture consists of equal masses of methane (molecular weight 16.0)and argon (atomic weight 40.0).If the partial pressure of argon is 200.torr,what is the pressure of methane,in torr?
A)80.0 torr
B)200.torr
C)256 torr
D)500.torr
E)556 torr
A)80.0 torr
B)200.torr
C)256 torr
D)500.torr
E)556 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
At what temperature in Kelvin is the root mean square speed of helium atoms (atomic weight = 4.00)equal to that of oxygen molecules (molecular weight = 32.00)at 300.K?
A)37.5 K
B)75 K
C)106 K
D)292 K
E)2400.K
A)37.5 K
B)75 K
C)106 K
D)292 K
E)2400.K

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
A compound composed of carbon,hydrogen,and chlorine effuses through a pinhole 0.411 times as fast as neon.Select the correct molecular formula for the compound.
A)CHCl3
B)CH2Cl2
C)C2H2Cl2
D)C2H3Cl
E)CCl4
A)CHCl3
B)CH2Cl2
C)C2H2Cl2
D)C2H3Cl
E)CCl4

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
Magnesium metal (0.100 mol)and a volume of aqueous hydrochloric acid that contains 0.500 mol of HCl are combined and react to completion.How many liters of hydrogen gas,measured at STP,are produced?
Mg(s)+ 2HCl(aq) MgCl2(aq)+ H2(g)
A)2.24 L of H2
B)4.48 L of H2
C)5.60 L of H2
D)11.2 L of H2
E)22.4 L of H2
Mg(s)+ 2HCl(aq) MgCl2(aq)+ H2(g)
A)2.24 L of H2
B)4.48 L of H2
C)5.60 L of H2
D)11.2 L of H2
E)22.4 L of H2

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following gases will be the slowest to diffuse through a room?
A)methane,CH4
B)hydrogen sulfide,H2S
C)carbon dioxide,CO2
D)water,H2O
E)neon,Ne
A)methane,CH4
B)hydrogen sulfide,H2S
C)carbon dioxide,CO2
D)water,H2O
E)neon,Ne

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
Arrange the following gases in order of increasing rate of effusion.
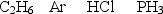
A)Ar < HCl3 < C2H6
B)C2H6 < PH3 < HCl < Ar
C)Ar < PH3 < C2H6 < HCl
D)C2H6 < HCl < PH3 < Ar
E)Ar3 < HCl < C2H6
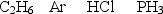
A)Ar < HCl
B)C2H6 < PH3 < HCl < Ar
C)Ar < PH3 < C2H6 < HCl
D)C2H6 < HCl < PH3 < Ar
E)Ar

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
Select the gas with the highest average kinetic energy per mole at 298 K.
A)O2
B)CO2
C)H2O
D)H2
E)All have the same average kinetic energy.
A)O2
B)CO2
C)H2O
D)H2
E)All have the same average kinetic energy.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
Lithium oxide is an effective absorber of carbon dioxide and can be used to purify air in confined areas such as space vehicles.What volume of carbon dioxide can be absorbed by 1.00 kg of lithium oxide at 25°C and 1.00 atm?
Li2O(aq)+ CO2(g) Li2CO3(s)
A)687 mL
B)819 mL
C)687 L
D)819 L
E)22.4 L
Li2O(aq)+ CO2(g) Li2CO3(s)
A)687 mL
B)819 mL
C)687 L
D)819 L
E)22.4 L

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
Hydrochloric acid is prepared by bubbling hydrogen chloride gas through water.What is the concentration of a solution prepared by dissolving 225 L of HCl(g)at 37°C and 89.6 kPa in 5.25 L of water?
A)1.49 M
B)1.66 M
C)7.82 M
D)12.5 M
E)16.6 M
A)1.49 M
B)1.66 M
C)7.82 M
D)12.5 M
E)16.6 M

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
A 3.0-L sample of helium was placed in a container fitted with a porous membrane.Half of the helium effused through the membrane in 24 hours.A 3.0-L sample of oxygen was placed in an identical container.How many hours will it take for half of the oxygen to effuse through the membrane?
A)8.5 h
B)12 h
C)48 h
D)60.h
E)68 h
A)8.5 h
B)12 h
C)48 h
D)60.h
E)68 h

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
Select the gas with the largest root-mean-square molecular speed at 25°C.
A)NH3
B)CO
C)H2
D)SF6
E)All the gases have the same root-mean-square molecular speed at 25°C.
A)NH3
B)CO
C)H2
D)SF6
E)All the gases have the same root-mean-square molecular speed at 25°C.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
Methane,CH4(g),reacts with steam to give synthesis gas,a mixture of carbon monoxide and hydrogen,which is used as starting material for the synthesis of a number of organic and inorganic compounds.
CH4(g)+ H2O(g) CO(g)+ H2(g)[unbalanced]
What mass of hydrogen is formed if 275 L of methane (measured at STP)is converted to synthesis gas?
A)12.3 g
B)24.7 g
C)37.1 g
D)49.4 g
E)74.2 g
CH4(g)+ H2O(g) CO(g)+ H2(g)[unbalanced]
What mass of hydrogen is formed if 275 L of methane (measured at STP)is converted to synthesis gas?
A)12.3 g
B)24.7 g
C)37.1 g
D)49.4 g
E)74.2 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
Hydrogen peroxide was catalytically decomposed and 75.3 mL of oxygen gas was collected over water at 25°C and 742 torr.What mass of oxygen was collected? (Pwater = 24 torr at 25°C)
A)0.00291 g
B)0.0931 g
C)0.0962 g
D)0.0993 g
E)0.962 g
A)0.00291 g
B)0.0931 g
C)0.0962 g
D)0.0993 g
E)0.962 g

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
Nitrogen will behave most like an ideal gas
A)at high temperature and high pressure.
B)at high temperature and low pressure.
C)at low temperature and high pressure.
D)at low temperature and low pressure.
E)at intermediate (moderate)temperature and pressure.
A)at high temperature and high pressure.
B)at high temperature and low pressure.
C)at low temperature and high pressure.
D)at low temperature and low pressure.
E)at intermediate (moderate)temperature and pressure.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
Use the van der Waals equation for real gases to calculate the pressure exerted by 1.00 mole of ammonia at 27°C in a 750-mL container.(a = 4.17 L2·atm/mol2,b = 0.0371 L/mol)
A)23.2 atm
B)27.1 atm
C)32.8 atm
D)42.0 atm
E)32.8 torr
A)23.2 atm
B)27.1 atm
C)32.8 atm
D)42.0 atm
E)32.8 torr

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
Dry air is approximately 78% nitrogen,21% oxygen and 1% argon,by number of molecules.What is the partial pressure of oxygen in a sample of dry air,if atmospheric pressure is 751 torr?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
A 1.00-L sample of a pure gas weighs 0.785 g and is at 0.965 atm and 29.2°C.
a.What is the molar mass of the gas?
b.If the volume and temperature are kept constant while 0.400 g of the same gas are added to that already in the container,what will the new pressure be?
a.What is the molar mass of the gas?
b.If the volume and temperature are kept constant while 0.400 g of the same gas are added to that already in the container,what will the new pressure be?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
Briefly state the conditions corresponding to STP (standard temperature and pressure).

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
A 255-mL gas sample weighing 0.292 g is at 52,810 Pa and 127°C.
a.How many moles of gas are present?
b.What is the molar mass of the gas?
a.How many moles of gas are present?
b.What is the molar mass of the gas?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
Calculate the density in g/L of gaseous SF6 at 50.0°C and 650.torr.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
The temperature of the carbon dioxide atmosphere near the surface of Venus is 475°C.Calculate the average kinetic energy per mole of carbon dioxide molecules on Venus.
A)2520 J/mol
B)4150 J/mol
C)5920 J/mol
D)9330 J/mol
E)5920 kJ/mol
A)2520 J/mol
B)4150 J/mol
C)5920 J/mol
D)9330 J/mol
E)5920 kJ/mol

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
State Boyle's Law and illustrate it with a graph,using standard x-y coordinate axes.Be sure to label the axes unambiguously with the correct gas variables.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
State Avogadro's Law.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
In the fermentation process,yeast converts glucose to ethanol and carbon dioxide.What volume of carbon dioxide,measured at 745 torr and 25.0°C,can be produced by the fermentation of 10.0 g of glucose?
C6H12O6(aq) 2C2H5OH(aq)+ 2CO2(g)
C6H12O6(aq) 2C2H5OH(aq)+ 2CO2(g)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
Aluminum metal shavings (10.0 g)are placed in 100.mL of 6.00 M hydrochloric acid.What is the maximum volume of hydrogen,measured at STP,which can be produced?
2Al(s)+ 6HCl(aq) 2AlCl3(aq)+ 3H2(g)
2Al(s)+ 6HCl(aq) 2AlCl3(aq)+ 3H2(g)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
A 3.60-L gas sample is at a pressure of 95.5 kPa and a temperature of 25.0°C.
a.Calculate the volume occupied by the gas at STP,assuming it behaves ideally.
b.If the gas sample weighs 6.10 g,calculate the molar mass of the gas.
a.Calculate the volume occupied by the gas at STP,assuming it behaves ideally.
b.If the gas sample weighs 6.10 g,calculate the molar mass of the gas.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
Starting from the Ideal Gas Equation,derive an equation corresponding to Charles' Law,stating all important assumptions or conditions.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
At moderate pressures (~ 200 atm),the measured pressure exerted by CO2 gas is less than that predicted by the ideal gas equation.This is mainly because
A)such high pressures cannot be accurately measured.
B)CO2 will condense to a liquid at 200 atm pressure.
C)gas phase collisions prevent CO2 molecules from colliding with the walls of the container.
D)of attractive intermolecular forces between CO2 molecules.
E)the volume occupied by the CO2 molecules themselves becomes significant.
A)such high pressures cannot be accurately measured.
B)CO2 will condense to a liquid at 200 atm pressure.
C)gas phase collisions prevent CO2 molecules from colliding with the walls of the container.
D)of attractive intermolecular forces between CO2 molecules.
E)the volume occupied by the CO2 molecules themselves becomes significant.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
A 1.30-L sample of argon gas is at 1.02 atm and 21.5°C.
a.What mass of argon is in the container?
b.If the temperature is raised to 500.0°C while the volume remains constant,calculate the new pressure,in atmospheres.
a.What mass of argon is in the container?
b.If the temperature is raised to 500.0°C while the volume remains constant,calculate the new pressure,in atmospheres.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
At very high pressures (~ 1000 atm),the measured pressure exerted by real gases is greater than that predicted by the ideal gas equation.This is mainly because
A)such high pressures cannot be accurately measured.
B)real gases will condense to form liquids at 1000 atm pressure.
C)gas phase collisions prevent molecules from colliding with the walls of the container.
D)of attractive intermolecular forces between gas molecules.
E)the volume occupied by the gas molecules themselves becomes significant.
A)such high pressures cannot be accurately measured.
B)real gases will condense to form liquids at 1000 atm pressure.
C)gas phase collisions prevent molecules from colliding with the walls of the container.
D)of attractive intermolecular forces between gas molecules.
E)the volume occupied by the gas molecules themselves becomes significant.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
An oxygen sample of 1.62 L is at 92.3 kPa and 30.0°C.
a.What is the pressure of oxygen,in torr?
b.What volume would the oxygen occupy if the pressure were 120.0 kPa and the temperature were 0.0°C?
c.How many moles of oxygen are in the sample?
a.What is the pressure of oxygen,in torr?
b.What volume would the oxygen occupy if the pressure were 120.0 kPa and the temperature were 0.0°C?
c.How many moles of oxygen are in the sample?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
Chlorofluorocarbons are important air pollutants implicated in global warming and ozone depletion.How fast does the chlorofluorocarbon CF2Cl2 diffuse relative to N2,the main component of the atmosphere? In other words,calculate the ratio of diffusion rates: 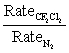
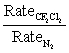

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
State Charles' Law and illustrate it with a graph,using standard x-y coordinate axes.Be sure to label the axes unambiguously with the correct gas variables.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck


