Deck 6: Chemical Composition
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 6: Chemical Composition
1
Avogadro's Number is 6.022 × 1023.
True
2
The lighter the atom,the less mass in one mole of that atom.
True
3
One mole of copper atoms is 6.022 × 1023 copper atoms.
True
4
One mole of nitrogen gas contains (2)× (6.022 × 1023)nitrogen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
The number 6.022 × 1023 is six times larger than the number 6.022 × 1022.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
The mole has a value of 6.023 × 1022.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
The chemical formula clearly indicates the relationship between the mass of each element in the formula.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
One mole of lead(II)nitrate contains six moles of oxygen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
Mass is used as a method of counting atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
The numerical value of the mole is defined as being equal to the number of atoms in exactly 12 grams of pure carbon-12.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
Six grams of carbon contains 3.008 × 1023 atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
The mass of 2.0 moles of H2O is greater than the mass of 1.0 mole of CO2.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
One mole of water contains 16 grams of oxygen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
One mole of zinc contains 65.39 zinc atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
One mole of chlorine gas has a mass of 35.45 grams.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
One mole of CO2 gas contains 1 mole of carbon atoms and 2 moles of oxygen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
Two moles of cobalt atoms have a mass of 117.87 grams.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
One mole of water contains 6.022 × 1023 hydrogen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
One mole of I2 has more atoms in it than one mole of Na.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
One mole of argon has more atoms in it than one mole of neon.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
The correct formula for calculating mass percent of X in compound XY is: 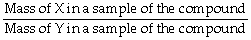
= Mass % X
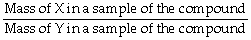
= Mass % X

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
Water is 11.2% hydrogen by mass.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
What is the correct value for Avogadro's number?
A)6.022 × 1023
B)6.022 × 1033
C)6.023 × 1022
D)6.022 × 102.3
E)none of the above
A)6.022 × 1023
B)6.022 × 1033
C)6.023 × 1022
D)6.022 × 102.3
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
The molar mass of a compound serves as a conversion factor between grams and moles.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
The empirical formula mass is 18.0 and the molecular formula mass is 90,therefore n = 5.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
The empirical formula mass must be 25.0 if the molecular formula mass is 250 and n = 5.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements about the mole is FALSE?
A)The size of a mole of atoms has a reasonable mass.
B)A mole of a monatomic element corresponds to one Avogadro's number of atoms.
C)One mole of a monatomic element has a mass equal to its atomic mass expressed in grams.
D)One mole of water contains 1/2 mole of oxygen atoms.
E)none of the above
A)The size of a mole of atoms has a reasonable mass.
B)A mole of a monatomic element corresponds to one Avogadro's number of atoms.
C)One mole of a monatomic element has a mass equal to its atomic mass expressed in grams.
D)One mole of water contains 1/2 mole of oxygen atoms.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
How many moles of Cu are in 1.48 × 1025 Cu atoms?
A)0.0408
B)24.6
C)1.54 × 1025
D)6.022 × 1023
E)none of the above
A)0.0408
B)24.6
C)1.54 × 1025
D)6.022 × 1023
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
C2H6O3 could be an empirical formula.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
C2H6O4 could be an empirical formula.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
The molecular formula is equal to the empirical formula multiplied by a whole number integer.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
An empirical formula gives the specific number of each type of atom in a molecule.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
An empirical formula gives the smallest whole number ratio of each type of atom in a molecule.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
How many atoms are in 1.50 moles of fluorine gas?
A)6.022 × 1023
B)9.03 × 1023
C)18.98
D)1.81 × 1024
E)none of the above
A)6.022 × 1023
B)9.03 × 1023
C)18.98
D)1.81 × 1024
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
A molecule that has an empirical formula of HO and a molar mass of 34.02 gram must have a molecular formula of H2O2.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
C2H3O2 could be an empirical formula.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
The empirical formula for C6H6 is C3H3.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
There are 6 grams of carbon in 22 grams of carbon dioxide.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
The chemical formula CuBr2 indicates that this compound is composed of 1 gram of copper and 2 grams of bromine.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
How many atoms are in 5.80 moles of He?
A)6.02 × 1023
B)1.03 × 1023
C)4.00
D)3.49 × 1024
E)none of the above
A)6.02 × 1023
B)1.03 × 1023
C)4.00
D)3.49 × 1024
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
What is the mass of 1.56 × 1021 atoms of magnesium in grams?
A)4.72 × 10-5
B)0.0630
C)0.142
D)1.07 × 10-4
E)none of the above
A)4.72 × 10-5
B)0.0630
C)0.142
D)1.07 × 10-4
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
What is the mass in grams of 5.40 moles of lithium?
A)6.94
B)37.5
C)1.29
D)3.25 × 1024
E)none of the above
A)6.94
B)37.5
C)1.29
D)3.25 × 1024
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
How many molecules of sulfur trioxide are in 78.0 grams?
A)5.87 × 1023
B)7.33 × 1023
C)3.76 × 1027
D)0.974
E)none of the above
A)5.87 × 1023
B)7.33 × 1023
C)3.76 × 1027
D)0.974
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
You have 10.0 g each of Na,C,Pb,Cu and Ne.Which contains the smallest number of moles?
A)Na
B)C
C)Pb
D)Cu
E)Ne
A)Na
B)C
C)Pb
D)Cu
E)Ne

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following contains 9.02 × 1023 atoms?
A)4.00 g H2
B)9.00 g H2O
C)28.0 g N2
D)32.0 g O2
E)none of the above
A)4.00 g H2
B)9.00 g H2O
C)28.0 g N2
D)32.0 g O2
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
In comparing 1 mole of carbon atoms to one mole of magnesium atoms,which statement is TRUE?
A)The mass of 1 mole of carbon is greater than the mass of 1 mole of magnesium.
B)The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon.
C)The mass of 1 mole of carbon is the same as the mass of 1 mole of magnesium.
D)There are more atoms in 1 mole of magnesium than in 1 mole of carbon.
E)none of the above
A)The mass of 1 mole of carbon is greater than the mass of 1 mole of magnesium.
B)The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon.
C)The mass of 1 mole of carbon is the same as the mass of 1 mole of magnesium.
D)There are more atoms in 1 mole of magnesium than in 1 mole of carbon.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
How many hydrogen atoms are in 35.0 grams of hydrogen gas?
A)4.25 × 1025
B)2.09 × 1025
C)2.12 × 1025
D)1.05 × 1025
E)none of the above
A)4.25 × 1025
B)2.09 × 1025
C)2.12 × 1025
D)1.05 × 1025
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
In comparing a balloon containing 25 grams of helium to a balloon containing 25 grams of neon,which one of the following statements is TRUE?
A)Each balloon has an equal number of atoms.
B)The helium balloon has more atoms.
C)The neon balloon has more atoms.
D)This scenario cannot happen because gases have no mass.
E)none of the above
A)Each balloon has an equal number of atoms.
B)The helium balloon has more atoms.
C)The neon balloon has more atoms.
D)This scenario cannot happen because gases have no mass.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
What is the mass of 3.09 × 1024 atoms of sulfur in grams?
A)9.64 × 1022
B)9.91 × 1025
C)165
D)0.160
E)none of the above
A)9.64 × 1022
B)9.91 × 1025
C)165
D)0.160
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
How many moles are there in 82.5 grams of iron?
A)4.97 × 1025
B)55.85
C)0.677
D)1.48
E)none of the above
A)4.97 × 1025
B)55.85
C)0.677
D)1.48
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles of iron are contained in 1.75 kg of iron?
A)3.13 × 10-2
B)3.13 × 10-4
C)31.3
D)3.13 × 104
E)none of the above
A)3.13 × 10-2
B)3.13 × 10-4
C)31.3
D)3.13 × 104
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
What is mass of 0.560 moles of chlorine gas?
A)19.9
B)63.3
C)127
D)39.7
E)none of the above
A)19.9
B)63.3
C)127
D)39.7
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
One mole of oxygen gas has a mass of ________ g.
A)16.0
B)32.0
C)6.022 × 1023
D)8
E)none of the above
A)16.0
B)32.0
C)6.022 × 1023
D)8
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
One mole of boron has a mass of ________ g.
A)9.012
B)6.022 × 1023
C)5
D)10.811
E)none of the above
A)9.012
B)6.022 × 1023
C)5
D)10.811
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
How many moles are there in 17.5 grams of sodium?
A)22.99
B)1.05 × 1025
C)0.761
D)1.31
E)none of the above
A)22.99
B)1.05 × 1025
C)0.761
D)1.31
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
You have 10.0 g each of Na,C,Pb,Cu and Ne.Which contains the largest number of moles?
A)Na
B)C
C)Pb
D)Cu
E)Ne
A)Na
B)C
C)Pb
D)Cu
E)Ne

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of Pb are in 4.71 × 1021 Pb atoms?
A)0.00782
B)2.84 × 1045
C)207.2
D)6.022 × 1023
E)none of the above
A)0.00782
B)2.84 × 1045
C)207.2
D)6.022 × 1023
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
How many moles of bromine gas are in 37.7 grams?
A)0.236
B)0.472
C)3.01 × 103
D)79.9
E)none of the above
A)0.236
B)0.472
C)3.01 × 103
D)79.9
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
The mass of one mole of carbon dioxide is ________ g.
A)28.01
B)384.4
C)32.00
D)44.01
E)none of the above
A)28.01
B)384.4
C)32.00
D)44.01
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
How many atoms are in 15.6 grams of silicon?
A)2.64 × 1026
B)3.34 × 1023
C)0.555
D)438
E)none of the above
A)2.64 × 1026
B)3.34 × 1023
C)0.555
D)438
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
Bauxite is an ore that contains the element aluminum.If you obtained 108 grams of aluminum from an ore sample that initially weighed 204 grams,what is the mass percent of aluminum in this bauxite ore?
A)52.9
B)15.6
C)0.53
D)47.1
E)none of the above
A)52.9
B)15.6
C)0.53
D)47.1
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
If 3.011 × 1023 molecules have a mass of 20.04 grams,what is the molar mass of this substance?
A)40.08 g/mol
B)10.02 g/mol
C)20.04 g/mol
D)6.658 × 10-23 g/mol
E)none of the above
A)40.08 g/mol
B)10.02 g/mol
C)20.04 g/mol
D)6.658 × 10-23 g/mol
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the molar mass of calcium nitrate.
A)136.03 g/mol
B)102.09 g/mol
C)132.10 g/mol
D)164.10 g/mol
E)none of the above
A)136.03 g/mol
B)102.09 g/mol
C)132.10 g/mol
D)164.10 g/mol
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
One mole of ammonium nitrite contains:
A)2 moles of nitrogen atoms.
B)4 moles of hydrogen atoms.
C)2 moles of oxygen atoms.
D)All of A,B,and C.
E)None of A,B,and C.
A)2 moles of nitrogen atoms.
B)4 moles of hydrogen atoms.
C)2 moles of oxygen atoms.
D)All of A,B,and C.
E)None of A,B,and C.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
How many moles of carbon are in 3.5 moles of calcium carbonate?
A)10.5
B)3.5
C)7
D)100.09
E)none of the above
A)10.5
B)3.5
C)7
D)100.09
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the molar mass of ammonium carbonate.
A)78.05 g/mol
B)88.05 g/mol
C)96.09 g/mol
D)112.09 g/mol
E)none of the above
A)78.05 g/mol
B)88.05 g/mol
C)96.09 g/mol
D)112.09 g/mol
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
What is the mass percent of chlorine in hydrochloric acid?
A)2.8
B)35.5
C)97.2
D)70.1
E)none of the above
A)2.8
B)35.5
C)97.2
D)70.1
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
How many molecules of nitrogen monoxide are in a 22.5 gram sample?
A)5.86 × 1023
B)7.33 × 1023
C)4.51 × 1023
D)4.06 × 1023
E)none of the above
A)5.86 × 1023
B)7.33 × 1023
C)4.51 × 1023
D)4.06 × 1023
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
If 2.01 × 1023 atoms of an element from Group IA of the periodic table has a mass of 7.675 grams,this element is most likely
A)Li.
B)Na.
C)K.
D)Rb.
E)Cs.
A)Li.
B)Na.
C)K.
D)Rb.
E)Cs.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
An iron ore sample is found to be 35.00% Fe by mass.How many grams of ore are needed to obtain 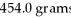
Of Fe?
A)1297
B)158.9
C)295.1
D)350.0
E)none of the above
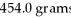
Of Fe?
A)1297
B)158.9
C)295.1
D)350.0
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
One mole of potassium sulfate contains:
A)4 moles of oxygen.
B)2 moles of sulfur.
C)1 mole of potassium.
D)3 moles of potassium.
E)none of the above
A)4 moles of oxygen.
B)2 moles of sulfur.
C)1 mole of potassium.
D)3 moles of potassium.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
One mole of (NH4)2HPO4 contains how many moles of hydrogen atoms?
A)4
B)2
C)8
D)9
E)none of the above
A)4
B)2
C)8
D)9
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
How many moles of fluorine are in 3.2 moles of xenon hexafluoride?
A)22.4
B)12.8
C)19.2
D)16
E)none of the above
A)22.4
B)12.8
C)19.2
D)16
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
What is the mass percent of hydrogen in water?
A)33.3
B)88.8
C)5.60
D)11.2
E)none of the above
A)33.3
B)88.8
C)5.60
D)11.2
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
If a sample of carbon dioxide contains 3.8 moles of oxygen atoms,how many moles of carbon dioxide are in the sample?
A)1.9
B)3.8
C)7.6
D)11.4
E)none of the above
A)1.9
B)3.8
C)7.6
D)11.4
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
One mole of ammonium nitrate contains:
A)3 moles of hydrogen.
B)2 moles of oxygen.
C)2 moles of nitrogen.
D)1 mole of nitrogen.
E)none of the above
A)3 moles of hydrogen.
B)2 moles of oxygen.
C)2 moles of nitrogen.
D)1 mole of nitrogen.
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
A 15.5 gram sample of diphosphorous pentoxide contains how many grams of phosphorous?
A)3.38
B)1.69
C)6.76
D)13.5
E)none of the above
A)3.38
B)1.69
C)6.76
D)13.5
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
A 500.gram iron ore sample was determined to contain 242 grams of iron.What is the mass percent of iron in the ore?
A)93.7
B)48.4
C)51.6
D)32.6
E)none of the above
A)93.7
B)48.4
C)51.6
D)32.6
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
A 42.7 gram sample of potassium nitrate contains how many grams of potassium?
A)39.1
B)16.5
C)21.4
D)8.54
E)none of the above
A)39.1
B)16.5
C)21.4
D)8.54
E)none of the above

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molar mass of aluminum sulfate?
A)123.0 g/mol
B)278.0 g/mol
C)306.2 g/mol
D)315.2 g/mol
E)342.2 g/mol
A)123.0 g/mol
B)278.0 g/mol
C)306.2 g/mol
D)315.2 g/mol
E)342.2 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


