Deck 13: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 13: Solutions
1
The minor component in a solution is called the solvent.
False
2
The solubility of gases in water decreases with increasing temperature.
True
3
Steel is an example of a solid solution.
True
4
The fact that the oceans contain salt water shows that polar solvents dissolve ionic solutes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Ionic solutes typically dissolve in nonpolar solvents.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Solubility is formally defined as the amount of a compound that can be dissolved in water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
The tragedy at Lake Nyos in Cameroon,West Africa,was due to the sudden release of excessive amounts of carbon monoxide dissolved in the lake water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
The major component in a solution is called the solute.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
Salt water is an example of a strong electrolyte solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
A solution is a homogeneous mixture of two or more substances.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
A sugar solution is an example of a weak electrolyte solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
The tragedy at Lake Nyos in Cameroon,West Africa was due to the sudden release of excessive amounts of nitrogen dissolved in the lake water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
The solubility of gases in water increases with increasing pressure above the water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
A saturated solution holds the maximum amount of solute under the solution conditions.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
When a chunk of gold is melted and poured into the shape of an ingot,an aqueous solution of gold has been created.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
The solubility of solids in water generally increases with increasing temperature.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
Warm beer goes flat quicker than cold beer.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Air is an example of a gaseous solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Tap water contains dissolved nitrogen and oxygen.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
A supersaturated solution is unstable and crystallization usually occurs.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
Osmosis is the process in which a solvent moves from an area of low solute concentration to an area of high solute concentration.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
A sample of salt water will freeze at a higher temperature than a sample of pure water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
A stock solution is a more concentrated form than what is typically used in a lab.It often requires further dilution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
A 0.10 molality solution of the sugar glucose (molecular weight = 180.10 g/mol)and a 0.10 molality solution of sucrose (molecular weight = 342.34 g/mol)would both boil at the same temperature.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
Osmotic pressure is the pressure required to completely reverse the direction of solvent movement in osmosis.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
Adding a nonvolatile solute to a liquid will cause boiling point depression and freezing point elevation.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following substances is NOT a solution?
A)air
B)brass
C)vodka
D)copper
E)All of the above are solutions.
A)air
B)brass
C)vodka
D)copper
E)All of the above are solutions.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
Sugar solutions conduct electricity because the dissolved particles are molecules.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
Molarity is defined as the moles of solute per liter of solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
Upon completing a dilution from a stock solution,you will always have more volume of solution than you started with and it will have a lower concentration than the stock solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
Colligative properties are independent of the amount of solute in solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
A solution that is 35 percent by mass NaCl contains 35 grams of NaCl dissolved in 100 grams of water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The molarity of a solution prepared by dissolving 15.0 grams of NaCl in 1000 mL water is 0.15 M.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
Molality is calculated by dividing grams of solute by kilograms of solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
If 50 grams of salt dissolves into 250 grams of water,the resulting solution must have a mass of 300 grams.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
Vitamin C is a water-soluble vitamin,so it is likely that this vitamin is polar.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
A solution that is 13.58 percent by mass of sugar contains 13.75 grams of sugar dissolved in 87.5 grams of water.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
One liter of 6.0 M HNO3 contains the same number of H+ ions as does one liter of 6.0 M H2SO4.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
A semipermeable membrane allows only half the amount of a substance to pass through it.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
The tragedy at Lake Nyos in Cameroon,West Africa,was caused by:
A)the release of excessive amounts of nitrogen gas that had been dissolved in the water at the bottom of the lake.
B)the release of excessive amounts of oxygen gas that had been dissolved in the water at the bottom of the lake.
C)the release of excessive amounts of carbon dioxide that had been dissolved in the water at the bottom of the lake.
D)the release of excess water stored in the volcanic lake which flooded the village below.
E)none of the above
A)the release of excessive amounts of nitrogen gas that had been dissolved in the water at the bottom of the lake.
B)the release of excessive amounts of oxygen gas that had been dissolved in the water at the bottom of the lake.
C)the release of excessive amounts of carbon dioxide that had been dissolved in the water at the bottom of the lake.
D)the release of excess water stored in the volcanic lake which flooded the village below.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
If the solubility of sodium acetate (Molar mass = 82 g/mol)is 76 grams per 100 grams of water,which of the following solutions would be considered supersaturated?
A)8.5 moles of sodium acetate dissolved in 1 L of water
B)5.5 moles of sodium acetate dissolved in 500 mL of water
C)1.8 moles of sodium acetate dissolved in 300 mL of water
D)1.2 moles of sodium acetate dissolved in 200 mL of water
E)none of the above
A)8.5 moles of sodium acetate dissolved in 1 L of water
B)5.5 moles of sodium acetate dissolved in 500 mL of water
C)1.8 moles of sodium acetate dissolved in 300 mL of water
D)1.2 moles of sodium acetate dissolved in 200 mL of water
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
The solubility of gases in water:
A)is independent of temperature.
B)increases with increasing temperature.
C)decreases with increasing temperature.
D)gases are not soluble in water.
E)none of the above
A)is independent of temperature.
B)increases with increasing temperature.
C)decreases with increasing temperature.
D)gases are not soluble in water.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
If the solubility of sodium chloride is 36 grams per 100 grams of water,which of the following solutions would be considered unsaturated?
A)5.8 moles of NaCl dissolved in 1 L of water
B)3.25 moles of NaCL dissolved in 500 ml of water
C)1.85 moles of NaCl dissolved in 300 ml of water
D)none of the above
A)5.8 moles of NaCl dissolved in 1 L of water
B)3.25 moles of NaCL dissolved in 500 ml of water
C)1.85 moles of NaCl dissolved in 300 ml of water
D)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following compounds is a strong electrolyte?
A)HCl
B)NaCl
C)NH4Cl
D)NaC2H3O2
E)all of the above
A)HCl
B)NaCl
C)NH4Cl
D)NaC2H3O2
E)all of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following substances is NOT a solution?
A)homogenized milk
B)bronze
C)sea water
D)soda
E)All of the above are solutions.
A)homogenized milk
B)bronze
C)sea water
D)soda
E)All of the above are solutions.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
When preparing sodium hydroxide solution,it is best to use water that does not contain any dissolved carbon dioxide as it reacts with the sodium hydroxide.Removing the carbon dioxide can be accomplished by:
A)vigorously stirring the solution.
B)using water fresh out of the purification system.
C)boiling the water.
D)Nothing can be done to remove dissolved gases.
E)none of the above
A)vigorously stirring the solution.
B)using water fresh out of the purification system.
C)boiling the water.
D)Nothing can be done to remove dissolved gases.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following substances is NOT a solution?
A)humid air
B)beer
C)oxygen
D)steel
E)All of the above are solutions.
A)humid air
B)beer
C)oxygen
D)steel
E)All of the above are solutions.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
The oxygen in the air we breath is classified as:
A)the solute in a homogeneous gas mixture.
B)the solvent in a homogeneous gas mixture.
C)the solute in a heterogeneous gas-liquid mixture.
D)the solvent in a simple mixture.
E)none of the above
A)the solute in a homogeneous gas mixture.
B)the solvent in a homogeneous gas mixture.
C)the solute in a heterogeneous gas-liquid mixture.
D)the solvent in a simple mixture.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
The solubility of a gas in a liquid can always be increased by:
A)increasing the temperature of the solvent.
B)decreasing the polarity of the solvent.
C)decreasing the pressure of the gas above the solvent.
D)increasing the pressure of the gas above the solvent.
E)none of the above
A)increasing the temperature of the solvent.
B)decreasing the polarity of the solvent.
C)decreasing the pressure of the gas above the solvent.
D)increasing the pressure of the gas above the solvent.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
Which among the following is NOT true about the solubility of a solid in water?
A)The solubility is not affected by pressure.
B)The solubility generally increases as temperature increases.
C)Solid crystallizes when a saturated solution is prepared at a higher temperature and then cooled.
D)A saturated solution prepared at a lower temperature becomes unsaturated when heated to a higher temperature.
E)none of the above
A)The solubility is not affected by pressure.
B)The solubility generally increases as temperature increases.
C)Solid crystallizes when a saturated solution is prepared at a higher temperature and then cooled.
D)A saturated solution prepared at a lower temperature becomes unsaturated when heated to a higher temperature.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
The solubility of solids in water:
A)is independent of the temperature.
B)increases with increasing temperature.
C)decreases with increasing temperature.
D)Solids are not soluble in water.
E)none of the above
A)is independent of the temperature.
B)increases with increasing temperature.
C)decreases with increasing temperature.
D)Solids are not soluble in water.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
Suppose a vodka martini contains 30% alcohol with the remaining portion of the drink composed of water.What is the solute in this type of martini?
A)water
B)alcohol
C)ice
D)olive
E)none of the above
A)water
B)alcohol
C)ice
D)olive
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds is a strong electrolyte?
A)C6H12O6
B)C7H14O5
C)C4H8O2
D)NaC2H3O2
E)all of the above
A)C6H12O6
B)C7H14O5
C)C4H8O2
D)NaC2H3O2
E)all of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
The solubility of Pb(NO3)2 is 55 grams per 100 g H2O at 20°C.Which term would properly describe a solution where 44 grams of Pb(NO3)2 is added to 100 grams of water at this temperature?
A)insoluble
B)unsaturated
C)saturated
D)supersaturated
E)none of the above
A)insoluble
B)unsaturated
C)saturated
D)supersaturated
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
Which of these compounds would you expect to be least soluble in water?
A)CH3OH
B)NaCl
C)N2
D)NH3
E)not enough information
A)CH3OH
B)NaCl
C)N2
D)NH3
E)not enough information

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
Hexane,a nonpolar solvent,will dissolve which of the following substances?
A)sodium chloride
B)oil
C)ammonium acetate
D)vinegar (acetic acid)
E)none of the above
A)sodium chloride
B)oil
C)ammonium acetate
D)vinegar (acetic acid)
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
In order for a solute to dissolve in solution:
A)the solute-solvent forces must be greater than the solute-solute forces.
B)the solute-solute forces must be greater than the solute-solvent forces.
C)the solute-solvent forces must equal the solute-solute forces.
D)the polarity of the solute and solvent must be opposite.
E)none of the above
A)the solute-solvent forces must be greater than the solute-solute forces.
B)the solute-solute forces must be greater than the solute-solvent forces.
C)the solute-solvent forces must equal the solute-solute forces.
D)the polarity of the solute and solvent must be opposite.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
If you prepare a solution by adding sufficient amount of solute so that after heating and cooling the solution there is a visible amount of solid solute left in the bottom of the beaker,the solution would be considered ________.
A)unsaturated.
B)saturated.
C)supersaturated.
D)thermally saturated.
E)none of the above
A)unsaturated.
B)saturated.
C)supersaturated.
D)thermally saturated.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
The solubility of solids in water:
A)is independent of pressure above solution.
B)increases with increasing pressure above solution.
C)decreases with increasing pressure above solution.
D)Solids are not soluble in water.
E)none of the above
A)is independent of pressure above solution.
B)increases with increasing pressure above solution.
C)decreases with increasing pressure above solution.
D)Solids are not soluble in water.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
Solubility of gases in water:
A)is independent of pressure above solution.
B)increases with increasing pressure above solution.
C)decreases with increasing pressure above solution.
D)Gases are not soluble in water.
E)none of the above
A)is independent of pressure above solution.
B)increases with increasing pressure above solution.
C)decreases with increasing pressure above solution.
D)Gases are not soluble in water.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
How many moles of NaF are in 34.2 grams of a 45.5% by mass NaF solution?
A)0.814
B)75.2
C)15.6
D)0.371
E)none of the above
A)0.814
B)75.2
C)15.6
D)0.371
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
Which solution below contains the highest total quantity of dissolved sodium ions?
A)100.mL of 4.0 M NaCl
B)75.0 mL of 3.0 M Na2SO4
C)50.0 mL of 8.0 M NaOH
D)50.0 mL of 2.0 M Na3PO4
E)none of the above
A)100.mL of 4.0 M NaCl
B)75.0 mL of 3.0 M Na2SO4
C)50.0 mL of 8.0 M NaOH
D)50.0 mL of 2.0 M Na3PO4
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
What is the mass percent of a solution prepared by dissolving 18.9 grams of solid into 39.5 grams of water?
A)47.8%
B)58.4%
C)32.4%
D)The identity of the compound must be known.
E)none of the above
A)47.8%
B)58.4%
C)32.4%
D)The identity of the compound must be known.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
What is the molarity of a solution prepared by dissolving 54.3 g of Ca(NO3)2 into 355 mL of water?
A)0.331 M
B)0.932 M
C)0.117 M
D)1.99 M
E)none of the above
A)0.331 M
B)0.932 M
C)0.117 M
D)1.99 M
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
How many moles of KOH are contained in 750.mL of 5.00 M KOH solution?
A)6.67 mol
B)3.75 ×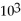
Mol
C)56.1 mol
D)3.75 mol
E)none of the above
A)6.67 mol
B)3.75 ×
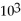
Mol
C)56.1 mol
D)3.75 mol
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
How many liters of a 2.18 M solution can be made from 200.0 g K2S?
A)0.832
B)1.81
C)0.252
D)1.20
E)none of the above
A)0.832
B)1.81
C)0.252
D)1.20
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
A solution contains 100.0 g water,10.0 g NaCl,and 15.0 g methanol.What is the weight percent of methanol in the solution?
A)8.00%
B)10.0%
C)12.0%
D)15.0%
E)none of the above
A)8.00%
B)10.0%
C)12.0%
D)15.0%
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
Given that you wished to use exactly 0.325 mole of NaCl to prepare a 2.50 M NaCl solution,how many milliliters of solution must you prepare?
A)130.mL
B)0.130 mL
C)7.69 mL
D)0.813 mL
E)none of the above
A)130.mL
B)0.130 mL
C)7.69 mL
D)0.813 mL
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
How many grams of KCl are needed to make 50.0 mL of 2.45 M KCl?
A)9.13
B)1.52
C)91.3
D)0.123
E)none of the above
A)9.13
B)1.52
C)91.3
D)0.123
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
We dissolve 2.45 g of sugar in 200.0 g water.What is the mass percent of sugar in the solution?
A)1.21%
B)1.23%
C)2.42%
D)123%
E)none of the above
A)1.21%
B)1.23%
C)2.42%
D)123%
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
How many grams of a 23.4% by mass NaF solution is needed if you want to have 1.33 moles of NaF?
A)55.9
B)31.1
C)13.1
D)239
E)none of the above
A)55.9
B)31.1
C)13.1
D)239
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
What are the ion concentrations in a 0.12 M solution of AlCl3?
A)0.36 M Al3+ ions and 0.12 M Cl- ions
B)0.12 M Al3+ ions and 0.040 M Cl- ions
C)0.12 M Al3+ ions and 0.36 M Cl- ions
D)0.040 M Al3+ ions and 0.040 M Cl- ions
E)none of the above
A)0.36 M Al3+ ions and 0.12 M Cl- ions
B)0.12 M Al3+ ions and 0.040 M Cl- ions
C)0.12 M Al3+ ions and 0.36 M Cl- ions
D)0.040 M Al3+ ions and 0.040 M Cl- ions
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
What is the molarity of a solution prepared by dissolving 10.7 g NaI in 0.250 L?
A)42.8
B)0.0714
C)2.86x 10-4
D)0.286
E)none of the above
A)42.8
B)0.0714
C)2.86x 10-4
D)0.286
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
What is the mass percent of an ammonium carbonate solution prepared by dissolving 33.2 grams of solid into 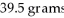
Of water?
A)84.1%
B)72.7%
C)45.7%
D)54.3%
E)none of the above
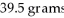
Of water?
A)84.1%
B)72.7%
C)45.7%
D)54.3%
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
A 0.15 M solution of BaCl2 contains:
A)0.15 M Ba2+ ions and 0.15 M Cl- ions.
B)0.15 M Ba2+ ions and 0.30 M Cl- ions.
C)0.30 M Ba2+ ions and 0.15 M Cl- ions.
D)0.30 M Ba2+ ions and 0.30 M Cl- ions.
E)none of the above
A)0.15 M Ba2+ ions and 0.15 M Cl- ions.
B)0.15 M Ba2+ ions and 0.30 M Cl- ions.
C)0.30 M Ba2+ ions and 0.15 M Cl- ions.
D)0.30 M Ba2+ ions and 0.30 M Cl- ions.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
What is the mass percent of a sodium fluoride solution prepared by dissolving 0.64 moles of sodium fluoride into 63.5 grams of water?
A)26.9%
B)42.3%
C)70.3%
D)29.7%
E)none of the above
A)26.9%
B)42.3%
C)70.3%
D)29.7%
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
After you have completed the task of diluting a solution,which statement below must be TRUE?
A)The new solution has more volume but has a lower concentration than before.
B)The new solution has more volume but has a higher concentration than before.
C)The new solution has less volume but has a lower concentration than before.
D)The new solution has less volume but has a higher concentration than before.
E)none of the above
A)The new solution has more volume but has a lower concentration than before.
B)The new solution has more volume but has a higher concentration than before.
C)The new solution has less volume but has a lower concentration than before.
D)The new solution has less volume but has a higher concentration than before.
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
You need to prepare 2.00 L of 0.100 M Na2CO3 solution.The best procedure is to weigh out
A)10.6 g Na2CO3 and add 2.00 L of water to it.
B)21.2 g Na2CO3 and add 2.00 L of water to it.
C)10.6 g Na2CO3 and add water until the final solution has a volume of 2.00 L.
D)21.2 g Na2CO3 and add water until the final solution has a volume of 2.00 L.
A)10.6 g Na2CO3 and add 2.00 L of water to it.
B)21.2 g Na2CO3 and add 2.00 L of water to it.
C)10.6 g Na2CO3 and add water until the final solution has a volume of 2.00 L.
D)21.2 g Na2CO3 and add water until the final solution has a volume of 2.00 L.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
A 90.0 g sample of NaOH is dissolved in water and the solution is diluted to give a final volume of 3.00 liters.The molarity of the final solution is ________.
A)0.500 M
B)0.750 M
C)1.00 M
D)2.25 M
E)none of the above
A)0.500 M
B)0.750 M
C)1.00 M
D)2.25 M
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
How many grams of LiF would be present in 575 mL of 0.750 M LiF solution?
A)11.2
B)0.0338
C)1.12 × 104
D)19.9
E)33.8
A)11.2
B)0.0338
C)1.12 × 104
D)19.9
E)33.8

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


