Deck 16: Oxidation and Reduction
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 16: Oxidation and Reduction
1
Reduction is the gain of electrons.
True
2
Fuel cells are based on the tendency of some elements to gain electrons from gasoline.
False
3
Redox reactions must involve the gain or loss of oxygen atoms.
False
4
The sum of the oxidation numbers of all atoms in K2Cr2O7 equals zero.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
The reducing agent is reduced during the reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction 2 NO2 → N2O4 would not be considered a redox reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
Given that the Activity Series shown below is accurate,then Li(s)+ K+(aq)→ Li+ (aq)+ K(s)is a spontaneous reaction.
Activity Series =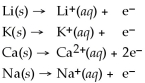
Activity Series =
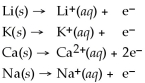

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
Cars are being developed that run on hydrogen gas and the only emission is water.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
Oxidation can be defined as the gain of oxygen atoms by another element.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
Reactions involving the transfer of electrons are called redox reactions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
The half-reaction 2 Br 1- → Br2 + 2 e- is balanced with respect to mass but it is not balanced with respect to charge.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
The oxidation number of manganese in MnO41- is +7.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
The oxidation number of a chlorine atom is -1.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
The oxidation number of sodium in NaI is +1.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
Oxidation and reduction cannot both occur in the same chemical reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
Oxidation is the loss of electrons.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
Reduction can be defined as the gain of oxygen atoms by another element.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
The oxidizing agent is the substance being reduced.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
The oxidation number of sulfur in SO3 is -6.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
Most acids dissolve metals by the oxidation of H+ ions to hydrogen gas.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
An electrolytic cell is a spontaneous redox reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
The rusting of iron is an example of the process known as corrosion.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
A spontaneous redox reaction can be used to produce electrical current.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
An electrochemical cell is based on the coupling of a spontaneous redox reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
A sacrificial electrode works by being reduced which prevents the metal from being oxidized.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
Oxidation occurs at the cathode.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
It can be shown that copper metal lies below H2 on the activity series of metals.This means that copper will dissolve in hydrochloric acid.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
The cathode is the electrode at which reduction occurs.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
Gold metal is found at the bottom of the activity series of metals since it is the most difficult metal to oxidize.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
The driving force that causes electrons to flow through a wire is called current.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
Corrosion of metals may be prevented by keeping them dry at all times.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
Rusting of iron requires the presence of both oxygen and water.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
Alkaline batteries are used in automobiles.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
To achieve the largest battery voltage possible,the battery should be assembled from a metal high on the activity series list with a metal ion low on the activity series list.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
Electrical current can be used to drive a nonspontaneous redox reaction.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
Which fact about fuel cells is FALSE?
A)Fuel cell automobiles are powered by water and the only emission is hydrogen.
B)Fuel cells are based on the tendency of some elements to gain electrons from other elements.
C)Fuel cell automobiles are whisper quiet.
D)Fuel cell automobiles are environmentally friendly.
E)all of the above
A)Fuel cell automobiles are powered by water and the only emission is hydrogen.
B)Fuel cells are based on the tendency of some elements to gain electrons from other elements.
C)Fuel cell automobiles are whisper quiet.
D)Fuel cell automobiles are environmentally friendly.
E)all of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Electrolysis is used to recover many metals from their oxide ores.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
Flashlight batteries are called dry cells.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
Ca2+ (aq)+ 2Na (s)→ Ca (s)+ 2 Na+ (aq)is a spontaneous reaction.
Activity Series =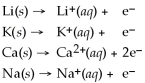
Activity Series =
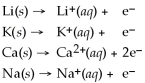

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
A galvanic cell is a spontaneous electrochemical cell.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
In the following reaction,
Mg (s)+ Cu2+ (aq)→ Mg2+ (aq)+ Cu (s):
A)Mg is the reducing agent and Cu is the oxidizing agent.
B)Mg2+ is the reducing agent and Cu is the oxidizing agent.
C)Cu is the reducing agent and Mg2+ is the oxidizing agent.
D)Cu2+ is the reducing agent and Mg is the oxidizing agent.
E)Mg is the reducing agent and Cu2+ is the oxidizing agent.
Mg (s)+ Cu2+ (aq)→ Mg2+ (aq)+ Cu (s):
A)Mg is the reducing agent and Cu is the oxidizing agent.
B)Mg2+ is the reducing agent and Cu is the oxidizing agent.
C)Cu is the reducing agent and Mg2+ is the oxidizing agent.
D)Cu2+ is the reducing agent and Mg is the oxidizing agent.
E)Mg is the reducing agent and Cu2+ is the oxidizing agent.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
For the reaction Si (s)+ O2 (g)→ SiO2 (g),the reducing agent is
A)Si.
B)O2.
C)SiO2.
D)O.
E)none of the above
A)Si.
B)O2.
C)SiO2.
D)O.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following are typically TRUE of a reducing agent?
1.It causes reduction.
2.It gains electron(s).
3.It is the oxidized substance.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2 or 3
1.It causes reduction.
2.It gains electron(s).
3.It is the oxidized substance.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2 or 3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
The reducing agent typically:
A)gains electrons.
B)always remains unchanged during a reaction.
C)is the oxidized substance.
D)is itself reduced.
E)none of the above
A)gains electrons.
B)always remains unchanged during a reaction.
C)is the oxidized substance.
D)is itself reduced.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
What is the oxidation state of the underlined element in the compound: CO2?
A)+1
B)+2
C)-2
D)+4
E)+6
A)+1
B)+2
C)-2
D)+4
E)+6

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the substance being reduced in the following reaction: CH4 + 2O2 CO2 + 2H2O.
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
Identify the oxidizing agent in the following reaction: CH4 + 2O2 CO2 + 2H2O.
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the reducing agent in the following reaction: CH4 + 2O2 CO2 + 2H2O.
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
Oxidation typically involves:
A)the loss of electrons.
B)the loss of oxygen.
C)the gain of electrons.
D)the gain of water.
E)none of the above
A)the loss of electrons.
B)the loss of oxygen.
C)the gain of electrons.
D)the gain of water.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
What is the oxidation state of the underlined element in the compound: H2SO4?
A)+1
B)+2
C)-2
D)+6
E)+4
A)+1
B)+2
C)-2
D)+6
E)+4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the substance being oxidized in the following reaction: CH4 + 2O2 CO2 + 2H2O.
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above
A)CH4
B)O2
C)CO2
D)H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
What is the oxidation state of the underlined element in the reaction: NaHCO3 + HCl NaCl + CO2 + H2O
A)0
B)+1
C)-1
D)+2
E)-2
A)0
B)+1
C)-1
D)+2
E)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
What is the oxidation state of the underlined element in the reaction: NaHCO3 + HCl NaCl + CO2 + H2O
A)0
B)+1
C)-1
D)+2
E)-2
A)0
B)+1
C)-1
D)+2
E)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
Reduction typically involves:
A)the loss of electrons.
B)the gain of oxygen.
C)the gain of electrons.
D)the gain of water.
E)none of the above
A)the loss of electrons.
B)the gain of oxygen.
C)the gain of electrons.
D)the gain of water.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
The oxidizing agent typically:
A)loses electrons.
B)gains oxygen.
C)is the reactant that is reduced.
D)is oxidized.
E)none of the above
A)loses electrons.
B)gains oxygen.
C)is the reactant that is reduced.
D)is oxidized.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
The oxidation number of Cr in Cr2O72- is
A)+2.
B)-2.
C)+7.
D)+6.
E)none of the above
A)+2.
B)-2.
C)+7.
D)+6.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
What is the oxidation state of the underlined element in the compound: H2SO4?
A)+1
B)+2
C)-2
D)+6
E)+4
A)+1
B)+2
C)-2
D)+6
E)+4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following are typically TRUE of an oxidizing agent?
1.It causes oxidation.
2.It gains electron(s).
3.It is the reduced substance.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3
1.It causes oxidation.
2.It gains electron(s).
3.It is the reduced substance.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
What is the oxidation state of the underlined element in the reaction: NaHCO3 + HCl NaCl + CO2 + H2O
A)0
B)+1
C)-1
D)+2
E)-2
A)0
B)+1
C)-1
D)+2
E)-2

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
In the following reaction,
Zn (s)+ CuSO4 (aq)→ ZnSO4 (aq)+ Cu (s)
A)Zn is the reducing agent and CuSO4 is the oxidizing agent.
B)CuSO4 is the reducing agent and Zn is the oxidizing agent.
C)ZnSO4 is the reducing agent and Cu is the oxidizing agent.
D)Cu2+ is the reducing agent and Zn2+ is the oxidizing agent.
E)none of the above
Zn (s)+ CuSO4 (aq)→ ZnSO4 (aq)+ Cu (s)
A)Zn is the reducing agent and CuSO4 is the oxidizing agent.
B)CuSO4 is the reducing agent and Zn is the oxidizing agent.
C)ZnSO4 is the reducing agent and Cu is the oxidizing agent.
D)Cu2+ is the reducing agent and Zn2+ is the oxidizing agent.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
Balance the following half reaction in base solution: H20 (l)→ H2 (g)
A)2H2O (l)+ 2 e- → H2 (g)+ 2 OH- (aq)
B)2H2O (l)→ 2H2 (g)+ 2 e- + O2
C)2H2O (l)→ H2 (g)+ 2 H+ + 2e-
D)H2O (l)→ 2H+ + 2OH-
E)2H2O (l)→ H2 (g)+ 2OH- (aq)+ 5 e-
A)2H2O (l)+ 2 e- → H2 (g)+ 2 OH- (aq)
B)2H2O (l)→ 2H2 (g)+ 2 e- + O2
C)2H2O (l)→ H2 (g)+ 2 H+ + 2e-
D)H2O (l)→ 2H+ + 2OH-
E)2H2O (l)→ H2 (g)+ 2OH- (aq)+ 5 e-

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
What is the oxidation state of nitrogen in N2O3?
A)0
B)+1
C)+2
D)+3
E)+6
A)0
B)+1
C)+2
D)+3
E)+6

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
The oxidation state of Na in Na2SO4 is:
A)+1
B)+2
C)+4
D)-1
E)none of the above
A)+1
B)+2
C)+4
D)-1
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
Which substance below would contain a nitrogen atom with the highest oxidation number of all those shown?
A)NH4 1+
B)N2
C)NO2
D)NO3 1-
E)NH3
A)NH4 1+
B)N2
C)NO2
D)NO3 1-
E)NH3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
Assign the oxidation state of each atom in potassium chlorate,KClO3.
A)K = +1,Cl = +1,O = -2
B)K = -1,Cl = -1,O = +2
C)K = +1,Cl = +5,O = -2
D)K = +1,Cl = -1,O = 0
E)K = -1,Cl = +1,O = 0
A)K = +1,Cl = +1,O = -2
B)K = -1,Cl = -1,O = +2
C)K = +1,Cl = +5,O = -2
D)K = +1,Cl = -1,O = 0
E)K = -1,Cl = +1,O = 0

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
If you properly balance the following half reaction in base solution,how many electrons would appear on the product side of the equation? Al (s)→ Al(OH)41- (aq)
A)2
B)3
C)4
D)5
E)none of the above
A)2
B)3
C)4
D)5
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
From the activity list included in this problem,which element/ion is the most easily oxidized?
Activity Series =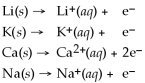
A)Na
B)Ca
C)Na+
D)Li
E)Li+
Activity Series =
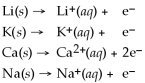
A)Na
B)Ca
C)Na+
D)Li
E)Li+

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
Reduction involves which of the following?
1.Loss of electron(s).
2.Gain of electron(s).
3.Decrease in oxidation state.
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)2 and 3 only
1.Loss of electron(s).
2.Gain of electron(s).
3.Decrease in oxidation state.
A)1 only
B)2 only
C)3 only
D)1 and 2 only
E)2 and 3 only

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
What is the balanced oxidation half-reaction for the following unbalanced redox reaction?
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2+ + 2 e-
B)Ag+ → Ag + e-
C)2 e- + Ca → Ca2+
D)2 Ag+ + 2 e-→ 2Ag
E)none of the above
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2+ + 2 e-
B)Ag+ → Ag + e-
C)2 e- + Ca → Ca2+
D)2 Ag+ + 2 e-→ 2Ag
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
Balance the following half reaction in acid solution: MnO4- Mn2+ (aq)
A)MnO4- → Mn2+ + 3 e-
B)MnO4- + 8 H+→ Mn2+ + 4 H2O
C)MnO4- + 8 H+→ Mn2+ + 4 H2O +5 e-
D)MnO4- + 8 H+ + 5 e-→ Mn2+ + 4 H2O
E)none of the above
A)MnO4- → Mn2+ + 3 e-
B)MnO4- + 8 H+→ Mn2+ + 4 H2O
C)MnO4- + 8 H+→ Mn2+ + 4 H2O +5 e-
D)MnO4- + 8 H+ + 5 e-→ Mn2+ + 4 H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
Assign the oxidation state of each atom in sodium sulfate,Na2SO4.
A)Na = +1,S = +4,O = -2
B)Na = +1,S = -2,O = +4
C)Na = +2,S = +6,O = -2
D)Na = +1,S = +6,O = -2
E)Na = +2,S = -2,O = 0
A)Na = +1,S = +4,O = -2
B)Na = +1,S = -2,O = +4
C)Na = +2,S = +6,O = -2
D)Na = +1,S = +6,O = -2
E)Na = +2,S = -2,O = 0

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
If you properly balance the following half reaction in acid solution,how many electrons would appear on the product side of the equation? NO (g)→ NO31- (aq)
A)1
B)2
C)3
D)5
E)none of the above
A)1
B)2
C)3
D)5
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
What is the oxidation state of the underlined element in the reaction: Cl2 + Mg MgCl2
A)0
B)+2
C)-2
D)+4
E)-4
A)0
B)+2
C)-2
D)+4
E)-4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
The oxidation state of Mn in KMnO4 is:
A)+1
B)-1
C)+5
D)+7
E)none of the above
A)+1
B)-1
C)+5
D)+7
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
What must be done to the following half-reactions before they can be added together?
Mn (s)→ Mn 2+(aq)+ 2e-
Fe 3+ (aq)+ 3 e- → Fe (s)
A)Double the Mn half-reaction.
B)Double the Mn half-reaction and triple the Fe half-reaction.
C)Triple the Mn half-reaction and double the Fe half-reaction.
D)Add H+ ions to the left-side of the Mn reaction.
E)none of the above
Mn (s)→ Mn 2+(aq)+ 2e-
Fe 3+ (aq)+ 3 e- → Fe (s)
A)Double the Mn half-reaction.
B)Double the Mn half-reaction and triple the Fe half-reaction.
C)Triple the Mn half-reaction and double the Fe half-reaction.
D)Add H+ ions to the left-side of the Mn reaction.
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
What is the balanced reduction half-reaction for the following unbalanced redox reaction:
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2++ 2 e-
B)Ag+ → Ag + 1 e-
C)2 e- + Ca → Ca2+
D)2Ag+ + 2 e-→ 2Ag
E)none of the above
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2++ 2 e-
B)Ag+ → Ag + 1 e-
C)2 e- + Ca → Ca2+
D)2Ag+ + 2 e-→ 2Ag
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
Balance the redox reaction: Br-(aq)+ Cr2O72-(aq) Cr3+ + Br2 (l)
A)4 Br- + Cr2O72- + 7 H+ → 2 Cr3+ + 2 Br2 + 7 H2O
B)6 Br- + Cr2O72- + 147 H+ → 2 Cr3+ + 3 Br2 + 7 H2O
C)2 Br-+ Cr2O72- + 147 H+ → 2 Cr3+ + Br2 + 7 H2O
D)2 Br- + 2 Cr2O72- + 147 H+ → 4 Cr3++ Br2 + 14 H2O
E)none of the above
A)4 Br- + Cr2O72- + 7 H+ → 2 Cr3+ + 2 Br2 + 7 H2O
B)6 Br- + Cr2O72- + 147 H+ → 2 Cr3+ + 3 Br2 + 7 H2O
C)2 Br-+ Cr2O72- + 147 H+ → 2 Cr3+ + Br2 + 7 H2O
D)2 Br- + 2 Cr2O72- + 147 H+ → 4 Cr3++ Br2 + 14 H2O
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
What is the oxidation state of sulfur in SO32-?
A)0
B)-2
C)+3
D)+4
E)+6
A)0
B)-2
C)+3
D)+4
E)+6

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
Oxidation involves which of the following?
1.Loss of electron(s).
2.Gain of electron(s).
3.Increase in oxidation state.
A)1 only
B)2 only
C)3 only
D)1 and 3 only
E)2 and 3 only
1.Loss of electron(s).
2.Gain of electron(s).
3.Increase in oxidation state.
A)1 only
B)2 only
C)3 only
D)1 and 3 only
E)2 and 3 only

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
What is the balanced oxidation half-reaction for the following unbalanced redox reaction:
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2+ + 2 e-
B)Ag+ → Ag + e-
C)2 e- + Ca → Ca2+
D)2 Ag+ + 2 e-→ 2Ag
E)none of the above
Ca(s)+ Ag+(aq) Ca2+(aq)+ Ag(s)
A)Ca → Ca2+ + 2 e-
B)Ag+ → Ag + e-
C)2 e- + Ca → Ca2+
D)2 Ag+ + 2 e-→ 2Ag
E)none of the above

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck


