Deck 17: Radioactivity and Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 17: Radioactivity and Nuclear Chemistry
1
When an atom emits a beta particle,its atomic number increases by one because it now has one additional proton.
True
2
The alpha particle (α)is: 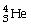
.
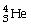
.
False
3
A gamma ray is a high energy photon.
True
4
Madam Curie discovered two new elements.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
All unstable elements have the same rates of radioactive decay.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
Radioactive particles can pass through matter.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
A nuclear reaction typically changes the identity of the element involved.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
During beta decay,neutrons are converted to protons and electrons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
When an atom emits an alpha particle,it becomes a different element.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
Nuclear equations do not need to be balanced since a new element forms.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
Antoine-Henri Becquerel discovered X-rays.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
A gamma ray has no charge and no mass.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
All elements with atomic numbers above bismuth are naturally radioactive.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
The symbol for a positron is 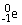
.
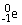
.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
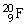
contains 11 protons and 9 neutrons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
Radioactivity is the emission of tiny,invisible radio signals by the nuclei of certain atoms.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
A positron particle comes from the decay of a proton into a neutron and a positron.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
In a Geiger-Muller counter,radioactive particles pass through NaI which emits UV-Vis light.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
A beta particle can also be called an electron.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
Alpha particles have lower penetrating power than the other types of radioactive emissions..

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
The effort to build an atomic bomb during WWII was prompted by a fear that Germany was already working on nuclear weapons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
A nuclide with a shorter half-life would be considered more active than a nuclide with a longer half-life.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
The hydrogen isotope known as tritium contains three neutrons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
Nuclear fission reactions produce ten times more energy per gram than fusion reactions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
A patient having radiotherapy uses radioactivity to kill infected cells while trying to minimize the effect on the healthy cells.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
One method of nuclear medicine uses radioactive isotopes to scan specific regions of the body.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
Nuclear power plants produce energy by fission reactions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
Radioactive elements are used for:
A)medical diagnosis.
B)medical treatment.
C)determining age of fossils and rocks.
D)generating electricity.
E)all of the above
A)medical diagnosis.
B)medical treatment.
C)determining age of fossils and rocks.
D)generating electricity.
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
Exposure to nuclear radioactivity can produce genetic defects in offspring.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
Who discovered radioactivity?
A)Antoine Becquerel
B)Pierre Curie
C)Marie Curie
D)Ernest Rutherford
E)Benjamin Franklin
A)Antoine Becquerel
B)Pierre Curie
C)Marie Curie
D)Ernest Rutherford
E)Benjamin Franklin

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
The energy of the sun comes from fusion reactions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
One danger of a nuclear power plant is that a chain reaction could produce a bomb.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
During the first half-life you lose half of the radioactive material,during the second half life you lose the remaining half.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
Uranium-235 is capable of undergoing a fission chain reaction.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
Radioactive elements become stable isotopes after emitting one of the radioactive particles 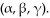

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
Nuclides that decay slowly have long half-lives.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
Carbon-14 dating is based on the fact that C-14 is continuously being made in the upper atmosphere.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
C-14 dating has been verified using old iron ore samples of known age.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
The combination of two light nuclei to form a heavier one is known as nuclear fission.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
A fusion reaction emits large amounts of energy while a fission reaction absorbs large amounts of energy.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
What happens to the mass number of a nucleus that emits an alpha particle?
A)It remains the same.
B)It decreases by two.
C)It decreases by four.
D)It increases by two.
E)It increases by four.
A)It remains the same.
B)It decreases by two.
C)It decreases by four.
D)It increases by two.
E)It increases by four.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
What is the main product when 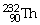
Undergoes beta decay?
A)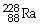
B)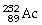
C)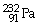
D)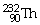
E)none of the above
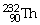
Undergoes beta decay?
A)
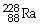
B)
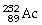
C)
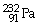
D)
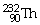
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following isotopes contains the most number of neutrons?
A)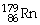
B)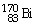
C)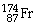
D)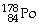
E)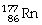
A)
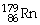
B)
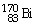
C)
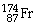
D)
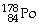
E)
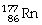

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following nuclides will undergo beta decay to produce 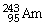
?
A)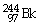
B)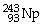
C)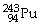
D)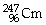
E)none of the above
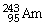
?
A)
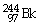
B)
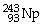
C)
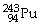
D)
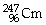
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
What is the main product when 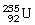
Undergoes beta decay?
A)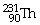
B)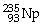
C)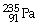
D)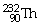
E)none of the above
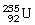
Undergoes beta decay?
A)
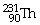
B)
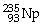
C)
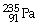
D)
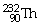
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements about beta particles is FALSE?
A)The symbol is: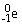
.
B)Beta particles are created when neutrons become protons and electrons.
C)They have intermediate ionizing power.
D)They have intermediate penetrating power.
E)They are a safe form of radioactivity.
A)The symbol is:
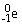
.
B)Beta particles are created when neutrons become protons and electrons.
C)They have intermediate ionizing power.
D)They have intermediate penetrating power.
E)They are a safe form of radioactivity.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
In a nuclear equation
A)the daughter nuclide appears on the right-side of the arrow.
B)the sum of the atomic numbers on both sides must be equal.
C)the sum of the mass numbers on both sides must be equal.
D)all of the above
E)none of the above
A)the daughter nuclide appears on the right-side of the arrow.
B)the sum of the atomic numbers on both sides must be equal.
C)the sum of the mass numbers on both sides must be equal.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following types of radiation has the highest penetrating power?
A)alpha particles
B)beta particles
C)gamma rays
D)positrons
E)none of the above
A)alpha particles
B)beta particles
C)gamma rays
D)positrons
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
During the career of Marie Curie,she:
A)won a Nobel prize in chemistry.
B)won a Nobel prize in physics.
C)discovered the element polonium.
D)discovered the element radium.
E)all of the above
A)won a Nobel prize in chemistry.
B)won a Nobel prize in physics.
C)discovered the element polonium.
D)discovered the element radium.
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
How was radioactivity discovered?
A)Rutherford was investigating the structure of Au atoms and discovered alpha particles.
B)Marie Curie was investigating the existence of uranic rays that occurred in some minerals.
C)Becquerel was studying X-rays and phosphorescence to see if they were related.
D)Benjamin Franklin was studying the creation of electrical current from the ionization of gases due to radioactive particles.
E)none of the above
A)Rutherford was investigating the structure of Au atoms and discovered alpha particles.
B)Marie Curie was investigating the existence of uranic rays that occurred in some minerals.
C)Becquerel was studying X-rays and phosphorescence to see if they were related.
D)Benjamin Franklin was studying the creation of electrical current from the ionization of gases due to radioactive particles.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
Which type of emission has a negative charge?
A)alpha
B)beta
C)positron
D)gamma
E)none of the above
A)alpha
B)beta
C)positron
D)gamma
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
How many protons and neutrons are in 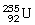
?
A)92 p and 235 n
B)92 n and 235 p
C)92 n and 143 p
D)92 p and 143 n
E)none of the above
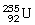
?
A)92 p and 235 n
B)92 n and 235 p
C)92 n and 143 p
D)92 p and 143 n
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement about alpha particles is FALSE?
A)symbol is: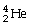
B)has high ionization power
C)has low penetrating power
D)are a harmless form of radiation
E)All of the above are true.
A)symbol is:
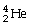
B)has high ionization power
C)has low penetrating power
D)are a harmless form of radiation
E)All of the above are true.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is NOT a type of radioactive emission?
A)alpha rays
B)beta rays
C)gamma rays
D)positrons
E)All of the above are types of radioactive emissions.
A)alpha rays
B)beta rays
C)gamma rays
D)positrons
E)All of the above are types of radioactive emissions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
How many protons and neutrons are in 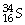
?
A)16 p and 34 n
B)16 n and 34 p
C)16 n and 18 p
D)16 p and 18 n
E)none of the above
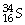
?
A)16 p and 34 n
B)16 n and 34 p
C)16 n and 18 p
D)16 p and 18 n
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
What happens to the atomic number of a nucleus that emits a beta particle?.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
What is the main product when 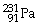
Undergoes alpha decay?
A)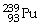
B)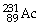
C)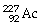
D)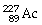
E)none of the above
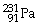
Undergoes alpha decay?
A)
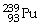
B)
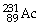
C)
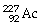
D)
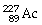
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
How many protons and neutrons are in 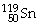
?
A)50 p and 169 n
B)50 n and 69 p
C)50 n and 119 p
D)50 p and 69 n
E)none of the above
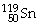
?
A)50 p and 169 n
B)50 n and 69 p
C)50 n and 119 p
D)50 p and 69 n
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
What is the main product when 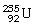
Undergoes alpha decay?
A)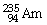
B)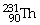
C)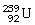
D)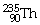
E)none of the above
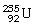
Undergoes alpha decay?
A)
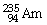
B)
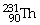
C)
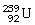
D)
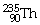
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
If the 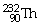
Isotope emits an alpha particle,what would be the atomic number of the
Resulting atom?
A)90
B)88
C)92
D)228
E)236
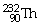
Isotope emits an alpha particle,what would be the atomic number of the
Resulting atom?
A)90
B)88
C)92
D)228
E)236

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
What is the missing particle? 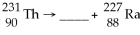
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
Tritium ( 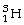
)is an isotope of hydrogen that is sometimes used to make the hands of watches glow in the dark.The half-life of tritium is 12.3 years.After 49 years,approximately how much of the original tritium remains?
A)50%
B)25%
C)12.5%
D)6.25%
E)3.12%
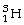
)is an isotope of hydrogen that is sometimes used to make the hands of watches glow in the dark.The half-life of tritium is 12.3 years.After 49 years,approximately how much of the original tritium remains?
A)50%
B)25%
C)12.5%
D)6.25%
E)3.12%

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
Radon-219 decays to radon-218 by releasing
A)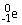
.
B)gamma rays.
C)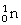
.
D)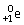
.
E)none of the above
A)
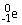
.
B)gamma rays.
C)
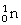
.
D)
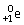
.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
The emitted particle with the mass of an electron but carrying a 1+ charge is named the
A)proton.
B)proelectron.
C)positron.
D)plusion.
E)none of the above
A)proton.
B)proelectron.
C)positron.
D)plusion.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
The rate of spontaneous nuclear decay:
A)can be increased by increasing the temperature.
B)can be increased by increasing the concentration of the radioactive element.
C)is independent of concentration or temperature.
D)can be increased by addition of a nuclear catalyst.
E)all of the above
A)can be increased by increasing the temperature.
B)can be increased by increasing the concentration of the radioactive element.
C)is independent of concentration or temperature.
D)can be increased by addition of a nuclear catalyst.
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
Which type of particle can be emitted by an unstable nucleus?
A)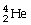
B)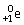
C)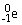
D)all of the above
E)none of the above
A)
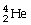
B)
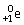
C)
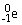
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
Which statement about positron emission is FALSE?
A)occurs when a proton is converted into a neutron and a positron
B)symbol is: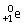
C)occurs with alpha and beta decay
D)alternative symbol is β+
E)All of the above are true.
A)occurs when a proton is converted into a neutron and a positron
B)symbol is:
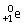
C)occurs with alpha and beta decay
D)alternative symbol is β+
E)All of the above are true.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
What is the missing particle? 
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
Atomic nuclei whose atomic number is greater than which element are considered to be radioactive?
A)Uranium
B)Radium
C)Barium
D)Bismuth
E)Lead
A)Uranium
B)Radium
C)Barium
D)Bismuth
E)Lead

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
What happens to the mass number of a nucleus that emits a positron?
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
What happens to the atomic number of nucleus that emits a positron?
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements regarding radiation are FALSE?
A)Radioactivity is a natural part of our environment.
B)All elements heavier than bismuth are radioactive.
C)All radioactive elements are spontaneously decaying towards formation of a stable element.
D)The time for half of the original sample to spontaneously decay is called the half-life (t1/2).
E)All of the above are true.
A)Radioactivity is a natural part of our environment.
B)All elements heavier than bismuth are radioactive.
C)All radioactive elements are spontaneously decaying towards formation of a stable element.
D)The time for half of the original sample to spontaneously decay is called the half-life (t1/2).
E)All of the above are true.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
What is the missing particle? 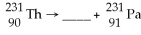
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
How does the emission of a gamma particle effect the radioactive atom?
A)The atom has a lower amount of energy.
B)The atomic number decreases.
C)The atomic mass increases.
D)The atom gains energy for further radioactive particle emission.
E)All of the above are true.
A)The atom has a lower amount of energy.
B)The atomic number decreases.
C)The atomic mass increases.
D)The atom gains energy for further radioactive particle emission.
E)All of the above are true.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
A scintillation counter detects radioactivity by:
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
A Geiger-Muller counter detects radioactivity by:
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
Which statement about gamma radiation is FALSE?
A)The symbol is: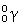
.
B)They have low ionization energy.
C)They have high penetrating power.
D)They are high energy photons.
E)All of the above are true.
A)The symbol is:
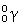
.
B)They have low ionization energy.
C)They have high penetrating power.
D)They are high energy photons.
E)All of the above are true.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
Iodine-131 has a half-life of 8 days.How much of a 1000 milligram sample of iodine-131 would be left after 32 days?
A)500 milligram
B)250 milligram
C)125 milligram
D)62.5 milligram
E)31.25 milligram
A)500 milligram
B)250 milligram
C)125 milligram
D)62.5 milligram
E)31.25 milligram

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
What type of radioactive decay produces a daughter nuclide that is the same element as the parent nuclide?
A)alpha
B)beta
C)gamma
D)positron
E)none of the above
A)alpha
B)beta
C)gamma
D)positron
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
If the half-life of a radioactive isotope is 32 days,how many half-lives must pass before a 3.0 gram sample is less than 0.03 g?
A)2
B)5
C)7
D)9
E)More information is needed.
A)2
B)5
C)7
D)9
E)More information is needed.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck


