Deck 14: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 14: Acids and Bases
1
The main component of vinegar is acetic acid.
True
2
Acids have a bitter taste.
False
3
The salt that forms due to neutralization of phosphoric acid by calcium hydroxide has the formula Ca3P2.
False
4
H⁺ is called the hydronium ion.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
The sour taste of bases warns humans against eating poisonous alkaloids found in some plants.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
NH4+ and NH3 are considered a conjugate acid-base conjugate pair.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
The conjugate base to HSO4- is SO42-.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
H2SO3 and H2SO4 are considered a conjugate acid-base pair.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
Bases feel slippery.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
The main component of stomach acid is sulfuric acid.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
The products of a neutralization reaction are carbon dioxide and water.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
Bases feel slippery because they react with oils on your skin to form soap-like substances.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
A Bronsted-Lowry acid is a proton donor.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
A conjugate acid-base pair are two substances related to each other by the transfer of a proton.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
Sodium hydroxide is often used in the manufacturing of soap.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
An Arrhenius base is a proton acceptor.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
When a metal reacts with an acid it produces salt and water.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
Bases have a bitter taste.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
Acids produce H⁺ ions in solution.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
Acids turn litmus paper blue.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
A solution that has a pH of 8.5 is considered a weak base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
Water always acts as an acid in reactions.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
A weak acid is a dilute acid that is not very powerful.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
The equation HCl (aq)+ H2O (l)↔ H3O+ (aq)+ Cl- (aq)properly depicts the behavior of hydrochloric acid in water.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
A solution of pH 2 contains ten times more H3O+ than a solution of pH 3.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
A solution with a concentration of 0.000 000 1 M HCl would be an acidic solution.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
Aluminum is one of the few metals that dissolves in a strong base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
A strong acid must also be a strong electrolyte.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
The ion product constant for water (Kw)is best stated as Kw = [H2O] [H+].

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
A 1.0 M [H3O+] solution of a strong acid would have a pH equal to zero.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
A 100 mL sample of 4.0 M H2SO4 could be neutralized by 100 mL of 4.0 M NH3.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
The pH of a 0.0001 M NaOH solution is 4.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
The pH of 0.001 M HCl is 3.0.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
In general,the stronger the acid,the weaker is its conjugate base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
A pH of 7 is equivalent to a pOH of 7.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
A strong acid is one that is very concentrated.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
Since diprotic acids such as H2CO3 have two ionizable protons,they will always behave as strong acids compared to monoprotic acids.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
A neutral solution does not contain any H⁺ or OH⁻.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
A strong acid is one that completely dissociates into ions in solution.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
In a titration,the indicator is used to signal when the endpoint has been reached.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
Which among the following acids is commonly used for etching and frosting glass?
A)hydrochloric acid
B)nitric acid
C)hydrofluoric acid
D)hydrobromic acid
E)All of the above are used.
A)hydrochloric acid
B)nitric acid
C)hydrofluoric acid
D)hydrobromic acid
E)All of the above are used.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
The Bronsted-Lowry definition of an acid is:
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
The Arrhenius definition of an acid is:
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following acids is commonly used for manufacturing fertilizer?
A)HCl
B)HN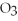
C)HF
D)HBr
E)all of the above
A)HCl
B)HN
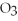
C)HF
D)HBr
E)all of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following common bases is commonly used for manufacturing fertilizer?
A)NH3
B)NaHCO3
C)KOH
D)NaOH
E)all of the above
A)NH3
B)NaHCO3
C)KOH
D)NaOH
E)all of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
A buffer resists a change in pH by being able to react with an acid or a base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
The burning of fossil fuels produces nitrogen oxides and sulfur oxides which can react to form acid rain (nitric and sulfuric acids).

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
What is the conjugate acid of OH⁻?
A)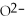
B)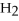
O
C)NaOH
D)OH⁻
E)none of the above
A)
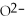
B)
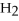
O
C)NaOH
D)OH⁻
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
A solution that has a pH of 10 would also have a pOH of 4.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is NOT a property of acids?
A)Acids have a slippery feel.
B)Acids have a sour taste.
C)Acids turn litmus paper red.
D)Acids dissolve many metals.
E)All of the above are properties of acids.
A)Acids have a slippery feel.
B)Acids have a sour taste.
C)Acids turn litmus paper red.
D)Acids dissolve many metals.
E)All of the above are properties of acids.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about acids are TRUE?
1.An acid is used in car batteries.
2.A component of vinegar is an acid.
3.Acids are used for cleaning metals.
A)1 and 2 only
B)2 and 3 only
C)1 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3
1.An acid is used in car batteries.
2.A component of vinegar is an acid.
3.Acids are used for cleaning metals.
A)1 and 2 only
B)2 and 3 only
C)1 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
A simple buffer can be prepared by mixing significant amounts of a weak acid and its conjugate base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements about a base are TRUE?
1.Bases are used in the manufacturing of soap.
2.Bases have a sour taste.
3.Fertilizer manufacture and cotton processing use bases.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3
1.Bases are used in the manufacturing of soap.
2.Bases have a sour taste.
3.Fertilizer manufacture and cotton processing use bases.
A)1 and 2 only
B)1 and 3 only
C)2 and 3 only
D)All of 1,2,and 3
E)Neither 1,2,or 3

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
Rain is naturally somewhat acidic because of atmospheric carbon dioxide.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
In examining the formula for acetic acid,HC2H3O2,the ionizable hydrogen atom(s)is/are:
A)the H on the left.
B)one of the H's on the right-side.
C)all of the H's on the right-side.
D)all four H's.
E)none of the above.
A)the H on the left.
B)one of the H's on the right-side.
C)all of the H's on the right-side.
D)all four H's.
E)none of the above.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
Carboxylic acids can be found in:
A)grapes.
B)apples.
C)lemons.
D)all of the above
E)none of these
A)grapes.
B)apples.
C)lemons.
D)all of the above
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is NOT true?
A)The Bronsted-Lowry Model applies to a wider range of acid-base phenomena than does the Arrhenius Model.
B)The Arrhenius Model of acids and bases was developed before the Bronsted-Lowry Model.
C)The Bronsted-Lowry Model can apply to bases that do not contain hydroxide ions.
D)The Arrhenius Model of acids and bases applies toward substances that are nonaqueous.
E)none of the above
A)The Bronsted-Lowry Model applies to a wider range of acid-base phenomena than does the Arrhenius Model.
B)The Arrhenius Model of acids and bases was developed before the Bronsted-Lowry Model.
C)The Bronsted-Lowry Model can apply to bases that do not contain hydroxide ions.
D)The Arrhenius Model of acids and bases applies toward substances that are nonaqueous.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is the active ingredient of baking soda?
A)NH3
B)NaHCO3
C)KOH
D)NaOH
E)none of the above
A)NH3
B)NaHCO3
C)KOH
D)NaOH
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
The Bronsted-Lowry definition of a base is:
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above
A)a proton donor.
B)a proton acceptor.
C)produces H⁺ in solution.
D)produces OH⁻ in solution.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is NOT a property of bases?
A)Bases have a slippery feel.
B)Bases have a bitter taste.
C)Bases turn litmus paper blue.
D)Bases dissolve many metals.
E)All of the above are properties of bases.
A)Bases have a slippery feel.
B)Bases have a bitter taste.
C)Bases turn litmus paper blue.
D)Bases dissolve many metals.
E)All of the above are properties of bases.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following acids is a monoprotic,strong acid?
A)sulfuric acid
B)phosphoric acid
C)hydrobromic acid
D)carbonic acid
E)none of the above
A)sulfuric acid
B)phosphoric acid
C)hydrobromic acid
D)carbonic acid
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
Exactly 17.0 mL of a H2SO4 solution was required to neutralize 45.0 mL of 0.235 M NaOH.What was the concentration of the H2SO4 solution?
Given: H2SO4 (aq)+ 2NaOH (aq)→ 2H2O (l)+ Na2SO4 (aq)
A)5.63 M
B)0.622 M
C)0.00529 M
D)0.311 M
E)none of the above
Given: H2SO4 (aq)+ 2NaOH (aq)→ 2H2O (l)+ Na2SO4 (aq)
A)5.63 M
B)0.622 M
C)0.00529 M
D)0.311 M
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
A neutralization reaction between an acid and sodium hydroxide formed water and the salt named sodium sulfate.What was the formula of the acid that was neutralized?
A)H2S
B)H2SO4
C)HCl
D)Na2SO4
E)none of the above
A)H2S
B)H2SO4
C)HCl
D)Na2SO4
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
What is the conjugate base of 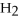
O?
A)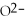
B)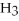
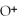
C)NaOH
D)OH⁻
E)none of the above
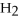
O?
A)
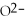
B)
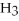
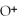
C)NaOH
D)OH⁻
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
In the following reaction: NH4+ (aq)+ H2O (aq)→ NH3 (aq)+ H3O+ (aq)
A)NH4+ is an acid and H2O is its conjugate base.
B)H2O is a base and NH3 is its conjugate acid.
C)NH4+ is an acid and H3O+ is its conjugate base.
D)H2O is a base and H3O+ is its conjugate acid.
E)NH4+ is a base and H2O is its conjugate acid.
A)NH4+ is an acid and H2O is its conjugate base.
B)H2O is a base and NH3 is its conjugate acid.
C)NH4+ is an acid and H3O+ is its conjugate base.
D)H2O is a base and H3O+ is its conjugate acid.
E)NH4+ is a base and H2O is its conjugate acid.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
In which solution is the [H3O+] less than 0.250 M?
A)0.250 M HC2H3O2 (aq)
B)0.250 M HF (aq)
C)0.250 M HCO2 (aq)
D)all of these
E)none of these
A)0.250 M HC2H3O2 (aq)
B)0.250 M HF (aq)
C)0.250 M HCO2 (aq)
D)all of these
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
Pure water cannot conduct electricity so why do we have to be careful with electrical appliances near water?
A)OSHA regulations
B)Water can be broken down by electricity.
C)Water always has some ions dissolved in it.
D)Water dissociates fully into its ions.
E)none of the above
A)OSHA regulations
B)Water can be broken down by electricity.
C)Water always has some ions dissolved in it.
D)Water dissociates fully into its ions.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
A 0.10 M solution of an electrolyte has a pH of 4.5.The electrolyte is:
A)a strong acid.
B)a strong base.
C)a weak acid.
D)a weak base.
E)none of the above
A)a strong acid.
B)a strong base.
C)a weak acid.
D)a weak base.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
When an acid reacts with a metal,what is one of the usual products?
A)water
B)salt
C)carbon dioxide
D)hydrogen gas
E)none of the above
A)water
B)salt
C)carbon dioxide
D)hydrogen gas
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is NOT an acid-base conjugate pair?
A)H2CO3 and HCO3-
B)H2O and OH-
C)H2S and OH-
D)NH4+ and NH3
E)none of the above
A)H2CO3 and HCO3-
B)H2O and OH-
C)H2S and OH-
D)NH4+ and NH3
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following acids is diprotic?
A)HCl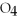
B)HN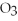
C)HI
D)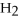
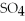
E)none of the above
A)HCl
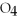
B)HN
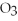
C)HI
D)
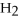
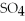
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following acids is a diprotic,weak acid?
A)sulfuric acid
B)phosphoric acid
C)hydrobromic acid
D)carbonic acid
E)none of the above
A)sulfuric acid
B)phosphoric acid
C)hydrobromic acid
D)carbonic acid
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
In the following reaction:
HCO3- (aq)+ H2O (aq)→ H2CO3 (aq)+ OH- (aq)
A)HCO3- is an acid and H2CO3 is its conjugate base.
B)H2O is an acid and OH- is its conjugate base.
C)HCO3- is an acid and OH- is its conjugate base.
D)H2O is an acid and H2CO3 is its conjugate base.
E)H2O is an acid and HCO3- is its conjugate base.
HCO3- (aq)+ H2O (aq)→ H2CO3 (aq)+ OH- (aq)
A)HCO3- is an acid and H2CO3 is its conjugate base.
B)H2O is an acid and OH- is its conjugate base.
C)HCO3- is an acid and OH- is its conjugate base.
D)H2O is an acid and H2CO3 is its conjugate base.
E)H2O is an acid and HCO3- is its conjugate base.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
A 25.0 mL sample of 0.105 M HCl was titrated with 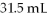
Of NaOH.What is the concentration of the NaOH?
A)0.0833 M
B)0.132 M
C)0.105 M
D)0.075 M
E)none of the above
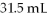
Of NaOH.What is the concentration of the NaOH?
A)0.0833 M
B)0.132 M
C)0.105 M
D)0.075 M
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
What are the products of a neutralization reaction?
A)salt and carbon dioxide
B)carbon dioxide and water
C)water and salt
D)oil and water
E)none of the above
A)salt and carbon dioxide
B)carbon dioxide and water
C)water and salt
D)oil and water
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
A substance that acts as an acid OR a base is called:
A)isoprotic.
B)a salt.
C)amphoteric.
D)hydrophillic.
E)none of the above
A)isoprotic.
B)a salt.
C)amphoteric.
D)hydrophillic.
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
A neutralization reaction between KOH (aq)and H2SO4 (aq)would give which two products?
A)H2O (l)and H2S (g)
B)H2O (l)and KSO4 (aq)
C)H2O (l)and K2SO4 (aq)
D)SO2 (g)and KH2 (g)
E)none of the above
A)H2O (l)and H2S (g)
B)H2O (l)and KSO4 (aq)
C)H2O (l)and K2SO4 (aq)
D)SO2 (g)and KH2 (g)
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
A 35.0 mL sample of 0.225 M HBr was titrated with 42.3 mL of KOH.What is the concentration of the KOH?
A)0.157 M
B)0.303 M
C)0.272 M
D)0.186 M
E)none of the above
A)0.157 M
B)0.303 M
C)0.272 M
D)0.186 M
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is a weak base?
A)calcium hydroxide
B)sodium fluoride
C)potassium hydroxide
D)ammonia
E)none of the above
A)calcium hydroxide
B)sodium fluoride
C)potassium hydroxide
D)ammonia
E)none of the above

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements about water are TRUE?
A)Water can act as an acid.
B)Water can act as a base.
C)The conjugate base of water is OH-.
D)The conjugate acid of water is H3O+.
E)All of the above are true.
A)Water can act as an acid.
B)Water can act as a base.
C)The conjugate base of water is OH-.
D)The conjugate acid of water is H3O+.
E)All of the above are true.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck


