Deck 11: Enzyme Catalysis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/53
Play
Full screen (f)
Deck 11: Enzyme Catalysis
1
An uncatalyzed reaction has a rate of 4.2 × 10-7 sec-1.When an enzyme is added the rate is 3.2 × 104 sec-1.Calculate the rate enhancement caused by the enzyme.
A)3)2 × 104
B)7)4 × 10-3
C)1)3 × 10-2
D)7)6 × 1010
E)The data are not appropriate for the calculation requested.
A)3)2 × 104
B)7)4 × 10-3
C)1)3 × 10-2
D)7)6 × 1010
E)The data are not appropriate for the calculation requested.
7)6 × 1010
2
Matching
-On a transition state diagram for a multistep reaction,the step with the greatest G is the ______.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
-On a transition state diagram for a multistep reaction,the step with the greatest G is the ______.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
rate-determining step
3
Matching
Metal ion cofactors can coordinate to the substrate or stabilize electrostatic effects by enabling proper ______in the active site.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Metal ion cofactors can coordinate to the substrate or stabilize electrostatic effects by enabling proper ______in the active site.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
orientation
4
Matching
If an enzyme-catalyzed reaction has a low rate at low pH and high rate at higher pH,this implies that a group on either the enzyme or the substrate must be ______ for an efficient reaction.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
If an enzyme-catalyzed reaction has a low rate at low pH and high rate at higher pH,this implies that a group on either the enzyme or the substrate must be ______ for an efficient reaction.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following is correct?
(I)All enzymes are highly specific for the reactions they catalyze.
(II)Prosthetic groups are loosely associated with the polypeptide chain of an enzyme.
(III)If an enzyme-catalyzed reaction requires a group with a low pK to be deprotonated and a group with a higher pK to be protonated,the pH vs.rate curve will have a peak in the middle of the two pK values.
(IV)When comparing types of catalysis,the proximity effect provides the largest rate enhancement.
A)I,II,III,IV
B)I,III
C)I
D)III
E)IV
(I)All enzymes are highly specific for the reactions they catalyze.
(II)Prosthetic groups are loosely associated with the polypeptide chain of an enzyme.
(III)If an enzyme-catalyzed reaction requires a group with a low pK to be deprotonated and a group with a higher pK to be protonated,the pH vs.rate curve will have a peak in the middle of the two pK values.
(IV)When comparing types of catalysis,the proximity effect provides the largest rate enhancement.
A)I,II,III,IV
B)I,III
C)I
D)III
E)IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following amino acid residues would not provide a side chain for acid-base catalysis at physiological pH? (Assume pK values of each amino acid are equal to the pK value for the free amino acid in solution. )
(I)leucine
(II)lysine
(III)aspartic acid
(IV)histidine
A)I,II,III
B)I,II
C)I
D)II
E)I,III
(I)leucine
(II)lysine
(III)aspartic acid
(IV)histidine
A)I,II,III
B)I,II
C)I
D)II
E)I,III

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
7
In affinity labeling,a technique used to study enzyme mechanisms,
A)A compound designed to bind at the active site reacts with and therefore permits the identification of a nearby group.
B)A fluorescent group is attached to the substrate to identify the position of the active site.
C)Highly purified enzyme is obtained by affinity chromatography for mechanistic studies.
D)A radioactive isotope is incorporated into the substrate to identify the position of the active site.
E)Genetic modification of amino acid residues is used to alter the binding affinity of the active site.
A)A compound designed to bind at the active site reacts with and therefore permits the identification of a nearby group.
B)A fluorescent group is attached to the substrate to identify the position of the active site.
C)Highly purified enzyme is obtained by affinity chromatography for mechanistic studies.
D)A radioactive isotope is incorporated into the substrate to identify the position of the active site.
E)Genetic modification of amino acid residues is used to alter the binding affinity of the active site.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
8
Matching
Some serine proteases are believed to have developed by convergent evolution,because the ______ sequences of some serine proteases show no resemblance to those of others.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Some serine proteases are believed to have developed by convergent evolution,because the ______ sequences of some serine proteases show no resemblance to those of others.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
9
Matching
Metal ion cofactors offer various ______ states that allow enzymes to accomplished oxidation reduction reactions.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Metal ion cofactors offer various ______ states that allow enzymes to accomplished oxidation reduction reactions.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
10
If you add enzyme to a solution containing only the product(s)of a reaction,would you expect any substrate to form?
A)It depends on the time interval and temperature of reaction.
B)It depends on the concentration of products added.
C)It depends on the energy difference between E + P and the transition state.
D)All of the above may determine if product forms.
E)None of the above determines if product forms.
A)It depends on the time interval and temperature of reaction.
B)It depends on the concentration of products added.
C)It depends on the energy difference between E + P and the transition state.
D)All of the above may determine if product forms.
E)None of the above determines if product forms.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
11
Matching
Since proteins are limited in their abilities to catalyze oxidation-reduction reactions,enzymes often employ ______ to assist with catalysis.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Since proteins are limited in their abilities to catalyze oxidation-reduction reactions,enzymes often employ ______ to assist with catalysis.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following amino acid residues would provide a side chain capable of increasing the hydrophobicity of a binding site?
A)histidine
B)lysine
C)isoleucine
D)arginine
E)serine
A)histidine
B)lysine
C)isoleucine
D)arginine
E)serine

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
13
The imidazole side chain of histidine can function as either a general acid catalyst or a general base catalyst because
(I)the imidazole group has a pKa in the physiological pH range.
(II)in the physiological pH range,the nitrogen in the ring can be easily protonated/deprotonated.
(III)one guanidino group is protonated,and the other is deprotonated at physiological pH.
(IV)the imidazole group is a strong reducing agent at physiological pH.
A)I,II
B)I
C)II,IV
D)II,III
E)IV
(I)the imidazole group has a pKa in the physiological pH range.
(II)in the physiological pH range,the nitrogen in the ring can be easily protonated/deprotonated.
(III)one guanidino group is protonated,and the other is deprotonated at physiological pH.
(IV)the imidazole group is a strong reducing agent at physiological pH.
A)I,II
B)I
C)II,IV
D)II,III
E)IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is TRUE about enzymes?
(I)Enzymes typically catalyze reactions at much higher rates than chemical catalyst.
(II)Enzymes are often very specific for their substrates.
(III)Enzyme activities can often be regulated.
(IV)Enzymes typically act under milder conditions of temperature and pH than chemical catalysts.
A)I,II,III
B)I,II,III,IV
C)II,II
D)III,IV
E)II,III,IV
(I)Enzymes typically catalyze reactions at much higher rates than chemical catalyst.
(II)Enzymes are often very specific for their substrates.
(III)Enzyme activities can often be regulated.
(IV)Enzymes typically act under milder conditions of temperature and pH than chemical catalysts.
A)I,II,III
B)I,II,III,IV
C)II,II
D)III,IV
E)II,III,IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
15
Matching
Large rate enhancement in enzyme catalysis may occur when binding the substrate via _________ attachment.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Large rate enhancement in enzyme catalysis may occur when binding the substrate via _________ attachment.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
16
Matching
-On a transition state diagram for a one-step very spontaneous reaction a large peak (high G‡)would imply a ______ rate for the reaction.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
-On a transition state diagram for a one-step very spontaneous reaction a large peak (high G‡)would imply a ______ rate for the reaction.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
17
Matching
Clustering several amino acid residues with appropriate pK values at an active site can promote a(n)_________ catalytic mechanism.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
Clustering several amino acid residues with appropriate pK values at an active site can promote a(n)_________ catalytic mechanism.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
18
A new serine protease was discovered that preferentially cleaves a peptide bond adjoining a negatively charged side chain.Which of the following is true?
A)The specificity pocket would mimic that of chymotrypsin.
B)The specificity pocket would mimic that of trypsin.
C)The specificity pocket is likely lined with amino acids such as Arg and Lys.
D)It likely reacts much slower than chymotrypsin.
E)It likely reaction much faster than chymotrypsin.
A)The specificity pocket would mimic that of chymotrypsin.
B)The specificity pocket would mimic that of trypsin.
C)The specificity pocket is likely lined with amino acids such as Arg and Lys.
D)It likely reacts much slower than chymotrypsin.
E)It likely reaction much faster than chymotrypsin.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these amino acid groups would not make a good nucleophilic catalyst?
A)amino
B)sulfhydryl
C)imidazole
D)methyl
E)hydroxyl
A)amino
B)sulfhydryl
C)imidazole
D)methyl
E)hydroxyl

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
20
Matching
For efficient nucleophilic catalysis,a group such as the sulfhydryl on a cysteine residue must be able to form a good ______,in addition to being a good nucleophile.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation
For efficient nucleophilic catalysis,a group such as the sulfhydryl on a cysteine residue must be able to form a good ______,in addition to being a good nucleophile.
A) high
B) deprotonated
C) protonated
D) catalytic mechanism
E) covalent
F) rate-determining step
G) leaving group
H) formation of ES
I) amino acid
J) low
K) coenzymes
L) concerted acid-base
M) orientation
N) oxidation

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
21
Carbohydrate metabolic enzymes bind D-glucose specifically.D-glucose has an estimated caloric value of 1 kcal per 4 grams of carbohydrate.Based on the methods used to convert the energy of D-glucose into a useable form,what would you estimate the caloric value of L-glucose to be using the same method?
A)1 kcal per 4 grams
B)1 kcal per 2 grams
C)1 kcal per 8 grams
D)0 kcal per 4 grams
E)2 kcal per 8 grams
A)1 kcal per 4 grams
B)1 kcal per 2 grams
C)1 kcal per 8 grams
D)0 kcal per 4 grams
E)2 kcal per 8 grams

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
22
Zymogens are not enzymatically active because
A)the active site amino acids have been mutated.
B)they have not yet bound the proper cofactor.
C)their environment has the wrong pH.
D)they are not yet shaped such that essential proximity and orientation catalysis can occur.
E)None of the above is correct.
A)the active site amino acids have been mutated.
B)they have not yet bound the proper cofactor.
C)their environment has the wrong pH.
D)they are not yet shaped such that essential proximity and orientation catalysis can occur.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the Schiff base formation reaction discussed in the catalytic mechanisms section of the chapter.What would probably happen if the carboxylate group was stabilized by a nearby positive charge?
A)The Schiff base would donate a lone pair of electrons to the substrate.
B)The Schiff base would form prior to decarboxylation.
C)The reaction would proceed with nucleophilic attack on the enzyme by the electrophilic substrate.
D)All of the above would be possible given the discussed mechanism with a stabilized carboxyl group.
E)None of the above is plausible considering the discussed mechanism with a stabilized carboxylate group.
A)The Schiff base would donate a lone pair of electrons to the substrate.
B)The Schiff base would form prior to decarboxylation.
C)The reaction would proceed with nucleophilic attack on the enzyme by the electrophilic substrate.
D)All of the above would be possible given the discussed mechanism with a stabilized carboxyl group.
E)None of the above is plausible considering the discussed mechanism with a stabilized carboxylate group.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is TRUE regarding cofactors?
A)Coenzymes are often separate from the enzyme and do not need recharged.
B)Metal ions must be covalently attached to function as a cofactor.
C)Cofactor is a broad term used for all enzyme "helpers".
D)Prosthetic groups can dissociate readily and be regenerated for use in another enzyme.
E)An apoenzyme implies that a cofactor is present.
A)Coenzymes are often separate from the enzyme and do not need recharged.
B)Metal ions must be covalently attached to function as a cofactor.
C)Cofactor is a broad term used for all enzyme "helpers".
D)Prosthetic groups can dissociate readily and be regenerated for use in another enzyme.
E)An apoenzyme implies that a cofactor is present.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
25
During the course of a catalytic reaction the following occurs: 1)Substrate is covalently bound and oriented with proximity to the active site residues.2)Negative charge builds up on the substrate and is stabilized.3)Oxidation of the enzyme followed by reduction to complete the catalytic cycle.What type of chemical species can facilitate these reactions?
A)nucleophilic amino acids
B)electrophilic groups
C)prosthetic phosphate groups
D)transition metal anions
E)transition metal cations
A)nucleophilic amino acids
B)electrophilic groups
C)prosthetic phosphate groups
D)transition metal anions
E)transition metal cations

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
26
A pH versus rate curve with an inflection point at pH~4 suggests the involvement of a(n)________ in the catalytic step.
A)proton abstraction that requires a metal ion in close proximity
B)proton transfer with a pK close to 4
C)proton donation that is mediated by a coenzyme
D)free proton surrounded by a hydrophobic environment
E)redox cofactor
A)proton abstraction that requires a metal ion in close proximity
B)proton transfer with a pK close to 4
C)proton donation that is mediated by a coenzyme
D)free proton surrounded by a hydrophobic environment
E)redox cofactor

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
27
Proton transfer from an acid,lowering the free energy of a reaction's transition state,is characteristic of
A)electrostatic catalysis.
B)nucleophilic catalysis.
C)general base catalysis.
D)general acid catalysis.
E)concerted acid-base catalysis.
A)electrostatic catalysis.
B)nucleophilic catalysis.
C)general base catalysis.
D)general acid catalysis.
E)concerted acid-base catalysis.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
28
Lysozyme requires _____ for effective catalysis.
(I)an Asp in a nonpolar environment
(II)a negatively charged Asp
(III)a Glu that functions as an acid catalyst
(IV)a Glu that is deprotonated
A)I,II,III,IV
B)I,III
C)II,III
D)II,IV
E)I,IV
(I)an Asp in a nonpolar environment
(II)a negatively charged Asp
(III)a Glu that functions as an acid catalyst
(IV)a Glu that is deprotonated
A)I,II,III,IV
B)I,III
C)II,III
D)II,IV
E)I,IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
29
In the lysozome reaction the D ring in NAM is in the ________ conformation providing a contribution of catalytic energy via the ____ distortion.
A)half-chair;electrostatic
B)chair;strain
C)boat;strain
D)boat;electrostatic
E)half-chair;strain
A)half-chair;electrostatic
B)chair;strain
C)boat;strain
D)boat;electrostatic
E)half-chair;strain

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
30
______ metals are the most common metallic enzyme cofactors.
A)Actinide
B)Alkali
C)Heavy
D)Rare earth
E)Transition
A)Actinide
B)Alkali
C)Heavy
D)Rare earth
E)Transition

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
31
Enzymes that bind reaction transition states with greater affinity than substrates or products
A)have a lower free energy ES complex than E + S.
B)increase reaction rates by decreasing the concentration of the transition state .
C)progress slowly due to the stability of the transition state complex.
D)compensate for small differences in the energy of the free substrate and free product.
E)All of the above are correct.
A)have a lower free energy ES complex than E + S.
B)increase reaction rates by decreasing the concentration of the transition state .
C)progress slowly due to the stability of the transition state complex.
D)compensate for small differences in the energy of the free substrate and free product.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
32
Glu 35 of lysozyme is found in a nonpolar environment.Which of the following is true?
A)Its pK is lower than the usual value in this environment.
B)Its pK is higher than the usual value in this environment.
C)Its pK would not change in this environment.
D)Its pK would depend on the sample buffer.
E)None of the above is correct.
A)Its pK is lower than the usual value in this environment.
B)Its pK is higher than the usual value in this environment.
C)Its pK would not change in this environment.
D)Its pK would depend on the sample buffer.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
33
Curved arrows are conventionally used to illustrate the movement of
A)electron pairs.
B)single electrons.
C)protons.
D)anions.
E)All of the above use this convention.
A)electron pairs.
B)single electrons.
C)protons.
D)anions.
E)All of the above use this convention.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following processes would yield and increase in rate?
A)the proximity of the reacting groups
B)the rotational motions of the substrates and catalytic groups
C)the orientations of the substrates and catalytic groups
D)all of the above
E)none of the above
A)the proximity of the reacting groups
B)the rotational motions of the substrates and catalytic groups
C)the orientations of the substrates and catalytic groups
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following would result in the greatest decrease in function of the catalytic triad found in serine proteases?
A)Mutation to Ser,Cys,Asp
B)Mutation to Ser,His,Asp
C)Mutation to Cys,His,Asp
D)Mutation to Ser,His,Glu
E)Mutation to Cys,His,Glu
A)Mutation to Ser,Cys,Asp
B)Mutation to Ser,His,Asp
C)Mutation to Cys,His,Asp
D)Mutation to Ser,His,Glu
E)Mutation to Cys,His,Glu

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
36
The catalytic mechanism of bovine pancreatic RNase A relies upon acid-base catalysis involving the amino acid __________.
A)imidazole
B)lysine
C)histidine
D)aspartic acid
E)glutamic acid
A)imidazole
B)lysine
C)histidine
D)aspartic acid
E)glutamic acid

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
37
Alcohol dehydrogenase catalyzes the conversion of methanol to _________ and is,therefore,classified as a(an)________.
A)acetic acid;transferase
B)formaldehyde;oxidoreductase
C)acetic acid;oxidoreductase
D)ethanol;lyase
E)formaldehyde;transferase
A)acetic acid;transferase
B)formaldehyde;oxidoreductase
C)acetic acid;oxidoreductase
D)ethanol;lyase
E)formaldehyde;transferase

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
38
Simultaneous stimulation of a reaction by general acid and general base catalysis is defined as
A)covalent catalysis.
B)electrostatic catalysis.
C)proximity catalysis.
D)concerted acid-base catalysis.
E)transition state catalysis.
A)covalent catalysis.
B)electrostatic catalysis.
C)proximity catalysis.
D)concerted acid-base catalysis.
E)transition state catalysis.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
39
Serine proteases use _________ to catalyze the cleavage of a peptide bond.
(I)covalent catalysis
(II)proximity and orientation effects
(III)general base catalysis
(IV)electrostatic catalysis
A)I,II
B)II
C)III
D)I,II,III
E)I,II,III,IV
(I)covalent catalysis
(II)proximity and orientation effects
(III)general base catalysis
(IV)electrostatic catalysis
A)I,II
B)II
C)III
D)I,II,III
E)I,II,III,IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
40
A peptide with the sequence "Glu-Ser-Val-Asp-Lys" will likely be cut next to "Val" rapidly by _______ and very slowly by ____.
(I)chymotrypsin
(II)trypsin
(III)trypsinogen
(IV)elastase
A)I,IV
B)I,II
C)II,III
D)IV,II
E)IV,I
(I)chymotrypsin
(II)trypsin
(III)trypsinogen
(IV)elastase
A)I,IV
B)I,II
C)II,III
D)IV,II
E)IV,I

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
41
Proximity effects
A)result from active site specificity.
B)result from substrate channeling.
C)result in increased effective concentration of substrate.
D)lower the energy of activation.
E)All of the above of the above are correct.
A)result from active site specificity.
B)result from substrate channeling.
C)result in increased effective concentration of substrate.
D)lower the energy of activation.
E)All of the above of the above are correct.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
42
Why can histidine NOT act as a base in the lysosome (an organelle where the pH is close to 4.5)?
A)Histidine has a positive charge at pH 4.5.
B)Histidine has a side chain pK close to 6.
C)Histidine would be protonated at pH 4.5.
D)All of the above are correct.
E)None of the above is correct.
A)Histidine has a positive charge at pH 4.5.
B)Histidine has a side chain pK close to 6.
C)Histidine would be protonated at pH 4.5.
D)All of the above are correct.
E)None of the above is correct.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is TRUE regarding transition state?
A)The free energy between the transition state and the reactants must be negative.
B)The transition state can be stabilized by covalent catalysis.
C)The transition state represents the ES complex.
D)Transition state analogs bind the substrate not the enzyme
E)All of the above are true.
A)The free energy between the transition state and the reactants must be negative.
B)The transition state can be stabilized by covalent catalysis.
C)The transition state represents the ES complex.
D)Transition state analogs bind the substrate not the enzyme
E)All of the above are true.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
44
A recent discovery has suggested that a gene mutation results in several amino acid substitutions within an active site.The following substitutions have been identified and are each expected to form the active site:
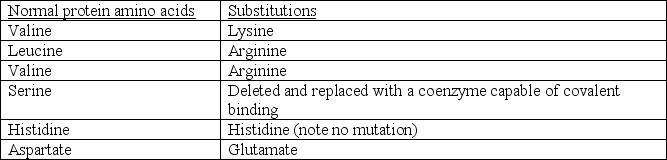
It was also noticed that a coenzyme molecule containing a hydroxyl (-OH)group binds to the mutated protein in approximately the same location as the original serine but does not alter the structure of the protein in anyway.Based on your knowledge of amino acids,enzymes,and catalysis,which of the following is a REASONABLE conclusion?
A)The mutant protein cleaves on the carboxy side of phenylalanine.
B)The mutant protein cleaves peptide bonds on the carboxy side of glutamate or aspartate.
C)The mutant protein cleaves peptide bonds on the carboxy side of lysine or arginine.
D)The mutant protein functions identical to chymotrypsin.
E)The mutant protein has no function.

It was also noticed that a coenzyme molecule containing a hydroxyl (-OH)group binds to the mutated protein in approximately the same location as the original serine but does not alter the structure of the protein in anyway.Based on your knowledge of amino acids,enzymes,and catalysis,which of the following is a REASONABLE conclusion?
A)The mutant protein cleaves on the carboxy side of phenylalanine.
B)The mutant protein cleaves peptide bonds on the carboxy side of glutamate or aspartate.
C)The mutant protein cleaves peptide bonds on the carboxy side of lysine or arginine.
D)The mutant protein functions identical to chymotrypsin.
E)The mutant protein has no function.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
45
Research scientists are trying to clone a gene.In order to accomplish this task they join two pieces of DNA.Which class of the enzymes below might accomplish this task?
A)oxidoreductase
B)ligase
C)hydrolase
D)isomerase
E)lyase
A)oxidoreductase
B)ligase
C)hydrolase
D)isomerase
E)lyase

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
46
In trypsin the specificity for one substrate over another comes from
A)the negatively charged pocket.
B)the positively charge pocket.
C)the hydrophobic pocket.
D)the amino acid serine.
E)the amino acid histidine.
A)the negatively charged pocket.
B)the positively charge pocket.
C)the hydrophobic pocket.
D)the amino acid serine.
E)the amino acid histidine.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
47
The transition state of an enzyme and substrate reaction
A)must always bind the enzyme active site with lower energy than the products.
B)is stabilized by enhancing the reverse reaction of E + S→ES.
C)is composed of true covalent bonds which decrease its energy.
D)is stabilized due to the specificity of the active site for the substrate.
E)is stabilized by decreasing the effective concentration.
A)must always bind the enzyme active site with lower energy than the products.
B)is stabilized by enhancing the reverse reaction of E + S→ES.
C)is composed of true covalent bonds which decrease its energy.
D)is stabilized due to the specificity of the active site for the substrate.
E)is stabilized by decreasing the effective concentration.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
48
Acid-base catalysis may be accomplished by:
A)active site specificity
B)charge delocalization
C)buffer effects
D)induced strain
E)conformational change
A)active site specificity
B)charge delocalization
C)buffer effects
D)induced strain
E)conformational change

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
49
The following diagram shows a portion of a reaction sequence of an enzyme.What type(s)of catalysis is (are)occurring in this first step? 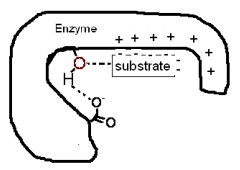
A)proximity catalysis
B)acid-base catalysis
C)covalent catalysis
D)All of the above are occurring.
E)None of the above is occurring.

A)proximity catalysis
B)acid-base catalysis
C)covalent catalysis
D)All of the above are occurring.
E)None of the above is occurring.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
50
Enzyme X catalyzes the addition of a hydroxyl group to substrate Y.In the process a metal cofactor is reduced and then reoxidized.What class of enzyme is X?
A)oxidoreductase
B)ligase
C)hydrolase
D)isomerase
E)lyase
A)oxidoreductase
B)ligase
C)hydrolase
D)isomerase
E)lyase

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
51
The enzyme pictured below is shown in two configurations,A (open)and B (closed).Upon binding substrate in the active site,the enzyme converts the structure shown in B.Which of the following is true based on the given information? 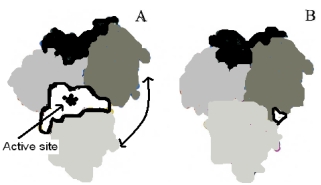
A)The enzyme requires a high concentration of substrate to function
B)This example exemplifies the "lock-and-key" model.
C)This enzyme must require ATP.
D)This example exemplifies the of "induced fit" model.
E)None of these are true.

A)The enzyme requires a high concentration of substrate to function
B)This example exemplifies the "lock-and-key" model.
C)This enzyme must require ATP.
D)This example exemplifies the of "induced fit" model.
E)None of these are true.

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
52
In the serine protease trypsin,the specificity for one substrate over another describes what type of catalysis?
A)proximity
B)acid-base
C)covalent
D)strain
E)none of the above
A)proximity
B)acid-base
C)covalent
D)strain
E)none of the above

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck
53
Which type of catalysis may be carried out using redistribution of electron density to facilitate the transfer of a proton?
(I)proximity
(II)acid-base
(III)covalent
(IV)strain
A)I
B)II
C)III
D)II,III
E)II,IV
(I)proximity
(II)acid-base
(III)covalent
(IV)strain
A)I
B)II
C)III
D)II,III
E)II,IV

Unlock Deck
Unlock for access to all 53 flashcards in this deck.
Unlock Deck
k this deck


