Deck 7: Chlorofluorocarbons and the Ozone Layer
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/29
Play
Full screen (f)
Deck 7: Chlorofluorocarbons and the Ozone Layer
1
What continent is the ozone hole located over?
A) Africa
B) Antarctica
C) Asia
D) North America
A) Africa
B) Antarctica
C) Asia
D) North America
Antarctica
2
What does the Chapman cycle illustrate?
A) the formation and destruction of water
B) the formation and destruction of carbon dioxide
C) the formation and destruction of nitrogen dioxide
D) the formation and destruction of ozone
A) the formation and destruction of water
B) the formation and destruction of carbon dioxide
C) the formation and destruction of nitrogen dioxide
D) the formation and destruction of ozone
the formation and destruction of ozone
3
Which type of radiation is the most energetic?
A) UV-A
B) UV-B
C) UV-C
D) visible
A) UV-A
B) UV-B
C) UV-C
D) visible
UV-C
4
What does one Dobson unit (DU) equal?
A) 1 ppb of ozone
B) 1 ppm of ozone
C) 100 ppb of ozone
D) 100 ppm of ozone
A) 1 ppb of ozone
B) 1 ppm of ozone
C) 100 ppb of ozone
D) 100 ppm of ozone

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
5
Which type of radiation is effective at damaging DNA?
A) UV-A
B) UV-B
C) UV-C
D) visible
A) UV-A
B) UV-B
C) UV-C
D) visible

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
6
Approximately how many ozone molecules can one chlorine atom derived from one CFC molecule react with?
A) 1 molecule O3
B) 100 molecules O3
C) 1000 molecules O3
D) 100000 molecules O3
A) 1 molecule O3
B) 100 molecules O3
C) 1000 molecules O3
D) 100000 molecules O3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following explains why CFCs are not removed from the atmosphere by rainout?
A) CFCs are not water soluble.
B) CRCs are chemically inactive in the troposphere.
C) UV radiation removes them more quickly.
D) This is due to both their chemical stability and solubility in water.
A) CFCs are not water soluble.
B) CRCs are chemically inactive in the troposphere.
C) UV radiation removes them more quickly.
D) This is due to both their chemical stability and solubility in water.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
8
In the past, which of the following was not a source of atmospheric CFCs?
A) air-conditioning
B) aerosol sprays
C) refrigerants
D) blown foams
E) All of the above have been sources of atmospheric CFCs.
A) air-conditioning
B) aerosol sprays
C) refrigerants
D) blown foams
E) All of the above have been sources of atmospheric CFCs.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
9
Which molecule is used as a replacement for CFC?
A) HFC-134a
B) HCFC-123
C) HFC-152a
D) all of the above
A) HFC-134a
B) HCFC-123
C) HFC-152a
D) all of the above

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
10
In a photochemical reaction,
A) the chemical reaction is energized by light.
B) nitrogen dioxide must be present.
C) ozone is always formed.
D) none of the above
A) the chemical reaction is energized by light.
B) nitrogen dioxide must be present.
C) ozone is always formed.
D) none of the above

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
11
Which type of chemical bond requires the least amount of energy to break?
A) single
B) double
C) triple
D) quadruple
A) single
B) double
C) triple
D) quadruple

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
12
Which molecule absorbs UV-B radiation?
A) O2
B) O3
C) CO2
D) NO2
A) O2
B) O3
C) CO2
D) NO2

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
13
Why are scientists worried about the ozone hole?
A) increase in skin cancer
B) increase in plant growth
C) decrease in ocean temperature
D) all of the above
A) increase in skin cancer
B) increase in plant growth
C) decrease in ocean temperature
D) all of the above

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
14
Chlorofluorocarbons, such as CCl2F2, can cause problems in the upper atmosphere by destroying
A) nitrogen oxides.
B) oxygen.
C) ozone.
D) polynuclear hydrocarbons.
A) nitrogen oxides.
B) oxygen.
C) ozone.
D) polynuclear hydrocarbons.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
15
What type of radiation is absorbed by molecular oxygen in the stratosphere?
A) UV-A
B) UV-B
C) UV-C
D) visible
A) UV-A
B) UV-B
C) UV-C
D) visible

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
16
Why must the ozone layer in the stratosphere be protected?
A) ozone poisons incoming, unwanted aliens
B) ozone absorbs some incoming ultraviolet light
C) ozone burns up incoming meteorites
D) ozone prevents oxygen from escaping the earth
A) ozone poisons incoming, unwanted aliens
B) ozone absorbs some incoming ultraviolet light
C) ozone burns up incoming meteorites
D) ozone prevents oxygen from escaping the earth

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
17
Light with a wavelength of 350 nm is classified as UV-A.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
18
UV-A radiation has a shorter wavelength than UV-B radiation.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
19
What chemical bond is broken by a photon of light in a typical CFC molecule?
A) C-H
B) Cl-Cl
C) H-Cl
D) C-Cl
A) C-H
B) Cl-Cl
C) H-Cl
D) C-Cl

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
20
Ozone-depleting substances typically contain which of the following elements?
A) oxygen
B) sulfur
C) nitrogen
D) chlorine
A) oxygen
B) sulfur
C) nitrogen
D) chlorine

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following sequence of four reactions. 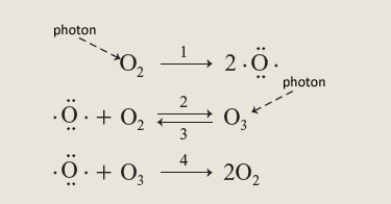 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
The reaction that is identical to a reaction in the cycle by which CFCs destroy ozone is reaction________.
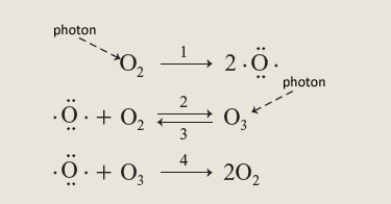 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.The reaction that is identical to a reaction in the cycle by which CFCs destroy ozone is reaction________.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
22
The following sequence of reactions describes the destruction of ozone in the troposphere. 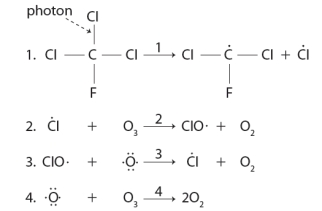
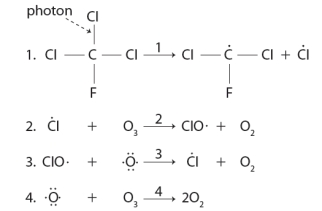

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
23
Decreasing ozone concentrations are only observed over Antarctica.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the following sequence of four reactions. 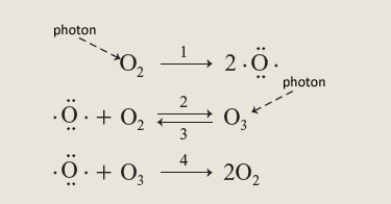 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
The reaction requiring a photon of the longest wavelength is reaction ______.
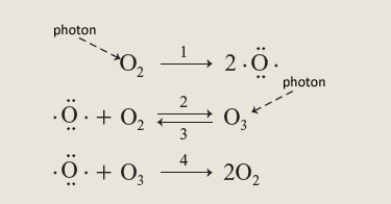 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.The reaction requiring a photon of the longest wavelength is reaction ______.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
25
More UV-A radiation reaches the Earth's surface than either UV-B or UV-C.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following sequence of four reactions. 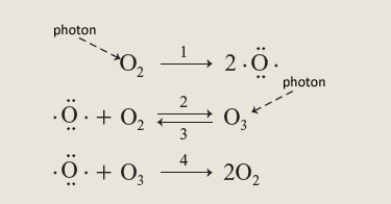 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
In order to attain a steady state one reaction must counteract another in a cycle such as that shown. A reaction which could be used to consume ozone is reaction_______.
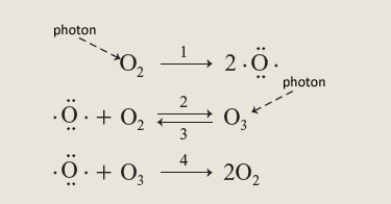 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.In order to attain a steady state one reaction must counteract another in a cycle such as that shown. A reaction which could be used to consume ozone is reaction_______.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the following sequence of four reactions. 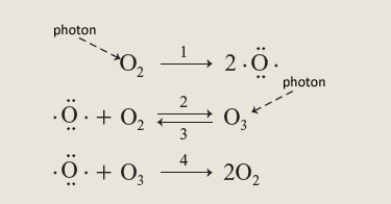 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
A reaction that would serve to decrease the amount of oxygen is reaction ________.
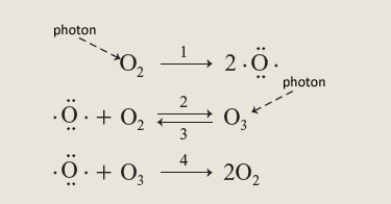 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.A reaction that would serve to decrease the amount of oxygen is reaction ________.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following sequence of reactions. 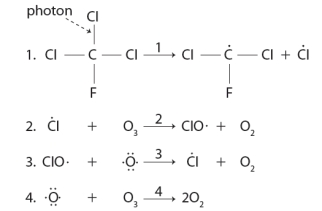 The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
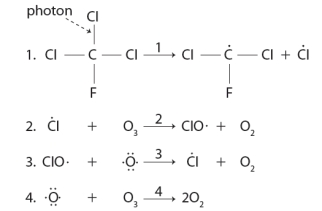 The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
The reason that low concentrations of CFCs can destroy major amounts of ozone is due to the production of a the reactive Cl species in this sequence.
Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the following sequence of four reactions. 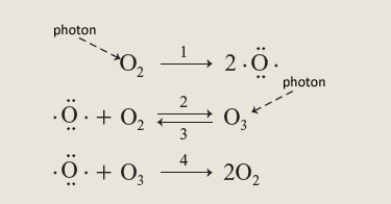 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
In order to attain a steady state one reaction must counteract another in a cycle such as that shown. The reaction which could be used to replace depleted ozone is reaction_______.
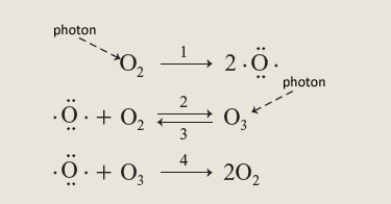 Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.
Answer the following question using the number of the appropriate reaction. More than one reaction may be applicable to a given question. You need only enter one reaction.In order to attain a steady state one reaction must counteract another in a cycle such as that shown. The reaction which could be used to replace depleted ozone is reaction_______.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck


