Deck 4: Water, Waves, and Tides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 4: Water, Waves, and Tides
1
Bases are compounds that do all the following except
A) they bind hydrogen ions.
B) they release hydrogen ions.
C) they are pH 7 or above.
D) they contain more hydroxide ions than hydrogen ions.
E) they raise pH.
A) they bind hydrogen ions.
B) they release hydrogen ions.
C) they are pH 7 or above.
D) they contain more hydroxide ions than hydrogen ions.
E) they raise pH.
B
2
Which low energy light wave length is quickly absorbed by water?
A) Red
B) Orange
C) Yellow
D) Green
E) a, b, and c above
A) Red
B) Orange
C) Yellow
D) Green
E) a, b, and c above
E
3
Surface tension allows water molecules to do all the following except:
A) transmit light energy.
B) resist evaporation.
C) form a tight surface layer.
D) support small organisms.
A) transmit light energy.
B) resist evaporation.
C) form a tight surface layer.
D) support small organisms.
A
4
Water can mingle with other elements because it is a:
A) heating agent.
B) solvent.
C) solid.
D) vapor.
E) nonpolar molecule.
A) heating agent.
B) solvent.
C) solid.
D) vapor.
E) nonpolar molecule.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
An ion is:
A) a type of gas.
B) an individually charged particle.
C) heavy water.
D) water with an extra hydrogen atom.
E) a neutrally-charged atom.
A) a type of gas.
B) an individually charged particle.
C) heavy water.
D) water with an extra hydrogen atom.
E) a neutrally-charged atom.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
You are SCUBA diving with a friend, who is wearing a red and purple wetsuit. You both descend and conduct your underwater research at 30 m. What color(s) does her wetsuit appear at this depth?
A) black and dark blue.
B) dark blue and purple.
C) dark green and black.
D) dark red and black.
A) black and dark blue.
B) dark blue and purple.
C) dark green and black.
D) dark red and black.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
Water is unique because its solid phase is ____ the liquid phase.
A) denser than
B) similar to
C) less dense than
A) denser than
B) similar to
C) less dense than

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
The specific heat of water is
A) a gram of substance.
B) energy required to raise a gram of substance 1 F.
C) calories need to heat seawater.
D) energy required to raise a gram of substance 1 C.
E) energy required to evaporate 1 gram of liquid water.
A) a gram of substance.
B) energy required to raise a gram of substance 1 F.
C) calories need to heat seawater.
D) energy required to raise a gram of substance 1 C.
E) energy required to evaporate 1 gram of liquid water.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
In most clear waters, 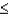 1% of surface light can penetrate to:
1% of surface light can penetrate to:
A) 10 m.
B) 20 m.
C) 50 m.
D) 75 m.
E) 100 m.
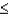 1% of surface light can penetrate to:
1% of surface light can penetrate to:A) 10 m.
B) 20 m.
C) 50 m.
D) 75 m.
E) 100 m.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
The property of water whereby molecules tend to stick to one another is called:
A) cohesion.
B) polarity.
C) dissolving ability.
D) adhesion.
E) viscosity.
A) cohesion.
B) polarity.
C) dissolving ability.
D) adhesion.
E) viscosity.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
The polarity of a water molecule is due to:
A) the greater number of hydrogen atoms relative to oxygen.
B) the uneven attraction of electrons to the oxygen atom.
C) the uneven attraction of electrons to the hydrogen atoms.
D) the uneven attraction of protons to the oxygen atom.
E) the number of neutrons in the nucleus.
A) the greater number of hydrogen atoms relative to oxygen.
B) the uneven attraction of electrons to the oxygen atom.
C) the uneven attraction of electrons to the hydrogen atoms.
D) the uneven attraction of protons to the oxygen atom.
E) the number of neutrons in the nucleus.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
Hydrogen forces have high attractive forces that allow water to have a high:
A) strength.
B) solubility.
C) freezing point.
D) boiling point.
E) rate of evaporation.
A) strength.
B) solubility.
C) freezing point.
D) boiling point.
E) rate of evaporation.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
The charge of a water molecule is considered:
A) polar.
B) positive.
C) negative.
D) neutral.
E) nonpolar.
A) polar.
B) positive.
C) negative.
D) neutral.
E) nonpolar.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
The property of water whereby molecules tend to stick to objects is called:
A) cohesion.
B) surface tension.
C) dissolving ability.
D) adhesion.
E) viscosity.
A) cohesion.
B) surface tension.
C) dissolving ability.
D) adhesion.
E) viscosity.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
Which high energy light wave length can penetrate sea water the deepest?
A) Blue
B) Green
C) Violet
D) Red
E) a, b, and c above
A) Blue
B) Green
C) Violet
D) Red
E) a, b, and c above

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
Hydrogen bonds are the result of attraction between:
A) the H of one water molecule and the H of another water molecule.
B) the two H atoms of the same water molecule.
C) the H and O of the same water molecule.
D) the H of one water molecule and the O of another water molecule.
A) the H of one water molecule and the H of another water molecule.
B) the two H atoms of the same water molecule.
C) the H and O of the same water molecule.
D) the H of one water molecule and the O of another water molecule.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
Acids are compounds that:
A) can bind hydrogen ions.
B) can release hydrogen ions.
C) are pH 8 or above.
D) contain hydrogen and hydroxide ions equal in number.
E) raise pH.
A) can bind hydrogen ions.
B) can release hydrogen ions.
C) are pH 8 or above.
D) contain hydrogen and hydroxide ions equal in number.
E) raise pH.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
Marine organisms contain ____ % water by mass.
A) 40-50
B) 50-60
C) 60-70
D) 70-80
E) 80-90
A) 40-50
B) 50-60
C) 60-70
D) 70-80
E) 80-90

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
Light that is absorbed by water is converted to:
A) short wave radiation.
B) long wave radiation.
C) heat.
D) mass.
E) green light.
A) short wave radiation.
B) long wave radiation.
C) heat.
D) mass.
E) green light.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
The unique attractive forces that keep molecules of water together are called:
A) strong forces.
B) adhesion.
C) hydrogen bonds.
D) cohesion.
E) weak nuclear forces.
A) strong forces.
B) adhesion.
C) hydrogen bonds.
D) cohesion.
E) weak nuclear forces.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
The maximum density of pure water occurs at:
A) -2° C.
B) 0° C.
C) 3° C.
D) 4° C.
E) -4° C.
A) -2° C.
B) 0° C.
C) 3° C.
D) 4° C.
E) -4° C.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
The mass of a substance divided by its volume is:
A) heat capacity.
B) surface tension.
C) density.
D) salinity.
E) viscosity.
A) heat capacity.
B) surface tension.
C) density.
D) salinity.
E) viscosity.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
A trace element's concentration is less than a part per
A) thousand.
B) million.
C) billion.
D) trillion.
E) hundred.
A) thousand.
B) million.
C) billion.
D) trillion.
E) hundred.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
Seawater is considered buffered due to the presence of:
A) oxygen ions.
B) nitrogen ions.
C) bicarbonate ions.
D) silicon ions.
E) calcium ions.
A) oxygen ions.
B) nitrogen ions.
C) bicarbonate ions.
D) silicon ions.
E) calcium ions.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
To remove carbon dioxide that is dissolved in seawater, you could ________the water sample.
A) chill
B) increase the pressure of
C) increase the salinity of
D) heat
A) chill
B) increase the pressure of
C) increase the salinity of
D) heat

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
The majority of the heat that is transferred from the earth to the atmosphere is transferred by:
A) radiation of heat into the atmosphere.
B) evaporation of water.
C) absorption by the lithosphere.
D) reflection of heat into space.
A) radiation of heat into the atmosphere.
B) evaporation of water.
C) absorption by the lithosphere.
D) reflection of heat into space.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
The salinity of seawater at the poles is:
A) high due to evaporation.
B) low due to precipitation.
C) high due to freezing.
D) low due to river input.
E) similar to the open ocean.
A) high due to evaporation.
B) low due to precipitation.
C) high due to freezing.
D) low due to river input.
E) similar to the open ocean.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
Water's pH is considered neutral when:
A) hydrogen can be bound.
B) hydrogen can be released.
C) water is slightly alkaline.
D) hydrogen and hydroxide ions are equal in number.
A) hydrogen can be bound.
B) hydrogen can be released.
C) water is slightly alkaline.
D) hydrogen and hydroxide ions are equal in number.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
The pH of seawater is important to living organisms because:
A) it can affect the functioning of enzymes.
B) it can interfere with metabolism.
C) it can affect growth.
D) All the above.
A) it can affect the functioning of enzymes.
B) it can interfere with metabolism.
C) it can affect growth.
D) All the above.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
The oxygen minimum layer is found
A) in the ocean trenches.
B) mid-ocean.
C) just below the sunlit surface.
D) at the surface.
E) just above the abyssal plain.
A) in the ocean trenches.
B) mid-ocean.
C) just below the sunlit surface.
D) at the surface.
E) just above the abyssal plain.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
The pH scale is a measure of:
A) how many degrees a professor has.
B) the temperature of water.
C) concentration of hydrogen ion in a volume of solution.
D) concentration of hydroxide ions in a volume of solution.
A) how many degrees a professor has.
B) the temperature of water.
C) concentration of hydrogen ion in a volume of solution.
D) concentration of hydroxide ions in a volume of solution.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
Salinity is expressed in parts per
A) hundred.
B) thousand.
C) ten thousand.
D) hundred thousand.
E) million.
A) hundred.
B) thousand.
C) ten thousand.
D) hundred thousand.
E) million.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
When a shallow extension of the sea dries out it leaves salt deposits called:
A) participates.
B) conglomerates.
C) evaporites.
D) rock salt.
E) sedimentary layers.
A) participates.
B) conglomerates.
C) evaporites.
D) rock salt.
E) sedimentary layers.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
The average salinity of seawater is ____0/00.
A) 35
B) 37
C) 40
D) 45
E) 50
A) 35
B) 37
C) 40
D) 45
E) 50

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
Salts are removed from the oceans primarily by
A) absorption by living organisms.
B) removal by sea spray.
C) evaporation.
D) adsorption onto particles.
A) absorption by living organisms.
B) removal by sea spray.
C) evaporation.
D) adsorption onto particles.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
The salinity of seawater at the equator is:
A) high due to evaporation.
B) low due to precipitation.
C) high due to freezing.
D) low due to river input.
A) high due to evaporation.
B) low due to precipitation.
C) high due to freezing.
D) low due to river input.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
The two main factors controlling the density of water are:
A) salinity and temperature.
B) salinity and heat capacity.
C) salinity and pH.
D) pH and heat capacity.
E) temperature and pH.
A) salinity and temperature.
B) salinity and heat capacity.
C) salinity and pH.
D) pH and heat capacity.
E) temperature and pH.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
The following are considered greenhouse gases except:
A) nitrogen.
B) carbon dioxide.
C) methane.
D) chlorofluorocarbons.
A) nitrogen.
B) carbon dioxide.
C) methane.
D) chlorofluorocarbons.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
In terms of gas solubility in sea water, what is the correct relationship?
A) Carbon dioxide > oxygen > nitrogen
B) Oxygen > carbon dioxide > nitrogen
C) Nitrogen > carbon dioxide > oxygen
D) Carbon dioxide > nitrogen > oxygen
E) Oxygen > nitrogen > carbon dioxide
A) Carbon dioxide > oxygen > nitrogen
B) Oxygen > carbon dioxide > nitrogen
C) Nitrogen > carbon dioxide > oxygen
D) Carbon dioxide > nitrogen > oxygen
E) Oxygen > nitrogen > carbon dioxide

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
A zone of rapid temperature change is called a(n):
A) pycnocline.
B) thermocline.
C) halocline.
D) isocline.
A) pycnocline.
B) thermocline.
C) halocline.
D) isocline.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
The capillary action of water is a result of the adhesive properties of water.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
The thermal capacity of water is due to the strong covalent bonds between the hydrogen and oxygen of a water molecule.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
Tsunamis are usually:
A) deep-water waves.
B) shallow-water waves.
C) wind waves.
D) capillary waves.
E) the result of winter storms.
A) deep-water waves.
B) shallow-water waves.
C) wind waves.
D) capillary waves.
E) the result of winter storms.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
On the pH scale, numbers below 7 indicate acidic solutions.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
The density of air increases with:
A) increasing temperature.
B) decreasing pressure.
C) increasing moisture.
D) decreasing moisture.
A) increasing temperature.
B) decreasing pressure.
C) increasing moisture.
D) decreasing moisture.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
A Class 4 hurricane is centered over Bermuda (latitude 32.20 N) and is moving eastwards. Which coastal city on the eastern coast of the United States is in the most peril from its approach?
A) Savannah, GA (latitude 32.08 N)
B) Jacksonville, FL (latitude 30.32 N)
C) Wilmington, NC (latitude 34.23 N)
D) Boston, MA (latitude 42.36 N)
A) Savannah, GA (latitude 32.08 N)
B) Jacksonville, FL (latitude 30.32 N)
C) Wilmington, NC (latitude 34.23 N)
D) Boston, MA (latitude 42.36 N)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
The Coriolis effect is:
A) the downwelling of seawater at the equator.
B) the upwelling of seawater at the equator.
C) the apparent deflection of the path of air and water.
D) the reduction of air speed over the ground as one goes from the equator to the poles.
A) the downwelling of seawater at the equator.
B) the upwelling of seawater at the equator.
C) the apparent deflection of the path of air and water.
D) the reduction of air speed over the ground as one goes from the equator to the poles.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
A common generating force for surface waves in the oceans is:
A) wind.
B) gravity.
C) surface tension.
D) capillary action.
E) undersea tectonic activity.
A) wind.
B) gravity.
C) surface tension.
D) capillary action.
E) undersea tectonic activity.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
A tidal pattern of two equal high and low tides per day is called a:
A) mixed semidiurnal tide.
B) semidiurnal tide.
C) diurnal tide.
D) spring tide.
E) neap tide.
A) mixed semidiurnal tide.
B) semidiurnal tide.
C) diurnal tide.
D) spring tide.
E) neap tide.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
Deep water waves move through water having a depth that is deeper than ____ the wavelength of the wave.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
The designation of winds is referenced from
A) the direction from which they are coming.
B) the direction towards which they are going.
C) folk lore.
D) the need to throw horses overboard.
E) the direction in relation to the observer's path of travel
A) the direction from which they are coming.
B) the direction towards which they are going.
C) folk lore.
D) the need to throw horses overboard.
E) the direction in relation to the observer's path of travel

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
A falling tide is called a(n):
A) flood tide.
B) spring tide.
C) neap tide.
D) ebb tide.
E) mixed tide.
A) flood tide.
B) spring tide.
C) neap tide.
D) ebb tide.
E) mixed tide.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
You are on the beach at Corpus Christi, Texas. High tide falls at noon. When will be the next high tide?
A) 6 pm that evening.
B) 12 hours later, at midnight.
C) 6 am the next morning.
D) noon the next day.
A) 6 pm that evening.
B) 12 hours later, at midnight.
C) 6 am the next morning.
D) noon the next day.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
Frozen water is more dense than liquid water.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
The surface tension of water is a result of the cohesive properties of water.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
Gyres are divided into the following types of currents (named below) except
A) Ekman spiral.
B) eastern boundary.
C) western boundary.
D) transverse.
A) Ekman spiral.
B) eastern boundary.
C) western boundary.
D) transverse.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
The distance over which wind is blowing is referred to as:
A) fetch.
B) duration.
C) strength.
D) depth.
E) wind span.
A) fetch.
B) duration.
C) strength.
D) depth.
E) wind span.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
An isopycnal water column is one in which the density ____ with depth.
A) increases
B) decreases
C) remains the same
D) None of the above.
A) increases
B) decreases
C) remains the same
D) None of the above.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
At 30° north and 30° south, air is:
A) cool and rising.
B) moist and rising.
C) moist and falling.
D) dry and falling.
E) dry and rising.
A) cool and rising.
B) moist and rising.
C) moist and falling.
D) dry and falling.
E) dry and rising.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
The charges on a water molecule are distributed equally.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
Plunging waves typically occur on very gently sloping coastlines.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
Trace elements are not important elements for living organisms.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
Match the Percentage by Volume in the Ocean with the gas.
a.83%
b.11%
c.6%
Oxygen
a.83%
b.11%
c.6%
Oxygen

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Trace elements dissolved in seawater are present in concentrations less than one part per thousand.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
As a wave approaches shallow water, its speed is reduced.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
MATCHING
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Oxygen
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Oxygen

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
The salinity of the world's oceans is increasing as more salts dissolve in the water.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
Air in the northern hemisphere is deflected to the left of its direction of movement due to the Coriolis effect.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
Oceanic surface currents are driven by winds.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
Capillary waves are small.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
Match the Percentage by Volume in the Ocean with the gas.
a.83%
b.11%
c.6%
Nitrogen
a.83%
b.11%
c.6%
Nitrogen

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
MATCHING
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Carbon dioxide
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Carbon dioxide

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
Match the Percentage by Volume in the Ocean with the gas.
a.83%
b.11%
c.6%
Carbon dioxide
a.83%
b.11%
c.6%
Carbon dioxide

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
Match the value of water with its property.
-Boiling Point
A)1.00 g/cm3
B)0 C
C)100 C
-Boiling Point
A)1.00 g/cm3
B)0 C
C)100 C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
Tsunamis are the result of the storm surge of hurricanes.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
MATCHING
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Nitrogen
Match the gas with the most closely associated atmospheric percentage.a.20.99%
b.0.03%
c.78.08%
Nitrogen

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Areas between wind convection cells are characterized by having stable and consistent winds.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
Match the value of water with its property.
-Freezing Point
A)1.00 g/cm3
B)0 C
C)100 C
-Freezing Point
A)1.00 g/cm3
B)0 C
C)100 C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
The concentration of CO2 in the atmosphere is lower than its concentration in the oceans.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
Surface temperature is more important than salinity in determining density of surface waters in coastal areas.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck


