Deck 2: The Components of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 2: The Components of Matter
1
Who is credited with discovering the atomic nucleus?
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
Rutherford
2
Who is credited with first measuring the charge of the electron?
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
Millikan
3
Bromine has two naturally-occurring isotopes. 79Br has a mass of 78.9 amu and accounts for 50.3% of bromine atoms. If the atomic mass of bromine is 79.9 amu, what is the mass of an atom of the second bromine isotope?
A) 77.9 amu
B) 80.0 amu
C) 80.1 amu
D) 80.9 amu
E) 88.9 amu
A) 77.9 amu
B) 80.0 amu
C) 80.1 amu
D) 80.9 amu
E) 88.9 amu
80.9 amu
4
What are the approximate carbon:hydrogen mass ratios in methane (CH4) and ethyne (C2H2)?
A) 1:4 and 1:1
B) 3:2 and 6:1
C) 3:1 and 12:1
D) 3:2 and 12:1
E) 3:1 and 6:1
A) 1:4 and 1:1
B) 3:2 and 6:1
C) 3:1 and 12:1
D) 3:2 and 12:1
E) 3:1 and 6:1

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
Millikan's oil-drop experiment
A) established the charge on an electron.
B) showed that all oil drops carried the same charge.
C) provided support for the nuclear model of the atom.
D) suggested that some oil drops carried fractional numbers of electrons.
E) suggested the presence of a neutral particle in the atom.
A) established the charge on an electron.
B) showed that all oil drops carried the same charge.
C) provided support for the nuclear model of the atom.
D) suggested that some oil drops carried fractional numbers of electrons.
E) suggested the presence of a neutral particle in the atom.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
The chemical symbol for potassium is
A) P.
B) Po.
C) Pt.
D) Pm.
E) K.
A) P.
B) Po.
C) Pt.
D) Pm.
E) K.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
In a Millikan oil-drop experiment, the charges on several different oil drops were as follows: -5.92; -4.44; -2.96; -8.88. The units are arbitrary. What is the likely value of the electronic charge in these arbitrary units?
A) -1.11
B) -1.48
C) -2.22
D) -2.96
E) -5.55
A) -1.11
B) -1.48
C) -2.22
D) -2.96
E) -5.55

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
Kaolinite, a clay mineral with the formula Al4Si4O10(OH)8, is used as a filler in slick-paper for magazines and as a raw material for ceramics. Analysis shows that 14.35 g of kaolinite contains 8.009 g of oxygen. Calculate the mass percent of oxygen in kaolinite.
A) 1.792 mass %
B) 24.80 mass %
C) 30.81 mass %
D) 34.12 mass %
E) 55.81 mass %
A) 1.792 mass %
B) 24.80 mass %
C) 30.81 mass %
D) 34.12 mass %
E) 55.81 mass %

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
An isotope of which of the following elements is chosen as a standard in measuring atomic mass?
A) carbon
B) oxygen
C) hydrogen
D) neon
E) helium
A) carbon
B) oxygen
C) hydrogen
D) neon
E) helium

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
When an atom is represented by the symbol 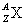 , the value of A is the
, the value of A is the
A) number of neutrons in the atom.
B) number of protons in the atom.
C) atomic mass of the element.
D) total number of electrons and neutrons in the atom.
E) total number of protons and neutrons in the atom.
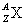 , the value of A is the
, the value of A is theA) number of neutrons in the atom.
B) number of protons in the atom.
C) atomic mass of the element.
D) total number of electrons and neutrons in the atom.
E) total number of protons and neutrons in the atom.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
Atoms X, Y, Z, and R have the following nuclear compositions:  Which two are isotopes?
Which two are isotopes?
A) X & Y
B) X & R
C) Y & R
D) Z & R
E) X & Z
 Which two are isotopes?
Which two are isotopes?A) X & Y
B) X & R
C) Y & R
D) Z & R
E) X & Z

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
Lithium forms compounds which are used in dry cells and storage batteries and in high-temperature lubricants. It has two naturally occurring isotopes, 6Li (isotopic mass = 6.015121 amu) and 7Li (isotopic mass = 7.016003 amu). Lithium has an atomic mass of 6.9409 amu. What is the percent abundance of lithium-6?
A) 92.50%
B) 86.66%
C) 46.16%
D) 7.503%
E) 6.080%
A) 92.50%
B) 86.66%
C) 46.16%
D) 7.503%
E) 6.080%

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
Bromine is the only nonmetal that is a liquid at room temperature. Consider the isotope bromine-81, 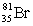 . Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
. Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
A) 35, 46, 81
B) 35, 81, 46
C) 81, 46, 35
D) 46, 81, 35
E) 35, 81, 116
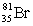 . Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
. Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.A) 35, 46, 81
B) 35, 81, 46
C) 81, 46, 35
D) 46, 81, 35
E) 35, 81, 116

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
Rutherford bombarded gold foil with alpha () particles and found that a small percentage of the particles were deflected. Which of the following was not accounted for by the model he proposed for the structure of atoms?
A) the small size of the nucleus
B) the charge on the nucleus
C) the total mass of the atom
D) the existence of protons
E) the presence of electrons outside the nucleus
A) the small size of the nucleus
B) the charge on the nucleus
C) the total mass of the atom
D) the existence of protons
E) the presence of electrons outside the nucleus

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
One amu is defined as
A) the mass of a proton.
B) 1/12 the mass of an atom of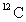 .
.
C) the mass of an atom of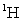 .
.
D) 1/20 the mass of an atom of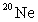 .
.
E) 1/16 the mass of an atom of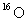 .
.
A) the mass of a proton.
B) 1/12 the mass of an atom of
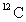 .
.C) the mass of an atom of
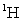 .
.D) 1/20 the mass of an atom of
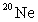 .
.E) 1/16 the mass of an atom of
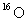 .
.
Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following statements about atoms and subatomic particles is correct?
A) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B) The proton and the neutron have identical masses.
C) The neutron's mass is equal to that of a proton plus an electron.
D) A neutral atom contains equal numbers of protons and electrons.
E) An atomic nucleus contains equal numbers of protons and neutrons.
A) Rutherford discovered the atomic nucleus by bombarding gold foil with electrons.
B) The proton and the neutron have identical masses.
C) The neutron's mass is equal to that of a proton plus an electron.
D) A neutral atom contains equal numbers of protons and electrons.
E) An atomic nucleus contains equal numbers of protons and neutrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
J. J. Thomson studied cathode ray particles (electrons) and was able to measure the mass/charge ratio. His results showed that
A) the mass/charge ratio varied with as the cathode material was changed.
B) the charge was always a whole-number multiple of some minimum charge.
C) matter included particles much smaller than the atom.
D) atoms contained dense areas of positive charge.
E) atoms are largely empty space.
A) the mass/charge ratio varied with as the cathode material was changed.
B) the charge was always a whole-number multiple of some minimum charge.
C) matter included particles much smaller than the atom.
D) atoms contained dense areas of positive charge.
E) atoms are largely empty space.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
In the modern periodic table, the order in which the elements are placed is based on
A) atomic mass.
B) mass number.
C) atomic number.
D) atomic size.
E) chemical reactivity.
A) atomic mass.
B) mass number.
C) atomic number.
D) atomic size.
E) chemical reactivity.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following symbols does not represent an element?
A) O2
B) Co
C) HF
D) Cs
E) Xe
A) O2
B) Co
C) HF
D) Cs
E) Xe

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
Who is credited with measuring the mass/charge ratio of the electron?
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford
A) Dalton
B) Gay-Lussac
C) Thomson
D) Millikan
E) Rutherford

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
The compound, BaO, absorbs water and carbon dioxide readily and is used to dry gases and organic solvents. What is its name?
A) barium oxide
B) barium(II) oxide
C) barium monoxide
D) baric oxide
E) barium peroxide
A) barium oxide
B) barium(II) oxide
C) barium monoxide
D) baric oxide
E) barium peroxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following ions occurs commonly?
A) N3+
B) S6+
C) O2-
D) Ca+
E) Cl+
A) N3+
B) S6+
C) O2-
D) Ca+
E) Cl+

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
A column of the periodic table is called a
A) group.
B) period.
C) isotopic mixture.
D) pillar.
E) shell.
A) group.
B) period.
C) isotopic mixture.
D) pillar.
E) shell.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
Sodium oxide combines violently with water. Which of the following gives the formula and the bonding for sodium oxide?
A) NaO, ionic compound
B) NaO, covalent compound
C) Na2O, ionic compound
D) Na2O, covalent compound
E) Na2O2, ionic compound
A) NaO, ionic compound
B) NaO, covalent compound
C) Na2O, ionic compound
D) Na2O, covalent compound
E) Na2O2, ionic compound

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a metalloid?
A) carbon, C, Z = 6
B) sulfur, S, Z = 16
C) germanium, Ge, Z = 32
D) iridium, Z = 77
E) bromine, Br, Z = 35
A) carbon, C, Z = 6
B) sulfur, S, Z = 16
C) germanium, Ge, Z = 32
D) iridium, Z = 77
E) bromine, Br, Z = 35

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
A row of the periodic table is called a
A) group.
B) period.
C) isotopic mixture.
D) family.
E) subshell.
A) group.
B) period.
C) isotopic mixture.
D) family.
E) subshell.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a non-metal?
A) lithium, Li, Z = 3
B) bromine, Br, Z = 35
C) mercury, Hg, Z = 80
D) bismuth, Bi, Z = 83
E) sodium, Na, Z = 11
A) lithium, Li, Z = 3
B) bromine, Br, Z = 35
C) mercury, Hg, Z = 80
D) bismuth, Bi, Z = 83
E) sodium, Na, Z = 11

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
Which, if any, of the following elements do not occur in the major classes of organic compounds?
A) H
B) C
C) N
D) O
E) All the above elements occur in the major classes of organic compounds.
A) H
B) C
C) N
D) O
E) All the above elements occur in the major classes of organic compounds.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a metal?
A) nitrogen, N, Z = 7
B) phosphorus, P, Z = 15
C) arsenic, Z = 33
D) thallium, Tl, Z = 81
E) silicon, Si, Z = 14
A) nitrogen, N, Z = 7
B) phosphorus, P, Z = 15
C) arsenic, Z = 33
D) thallium, Tl, Z = 81
E) silicon, Si, Z = 14

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
After an atom has lost an electron it becomes a/an ______ and has a _______ charge.
A) anion, positive
B) isotope, negative
C) anion, negative
D) cation, positive
E) nucleus, positive
A) anion, positive
B) isotope, negative
C) anion, negative
D) cation, positive
E) nucleus, positive

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds is ionic?
A) PF3
B) CS2
C) HCl
D) SO2
E) MgCl2
A) PF3
B) CS2
C) HCl
D) SO2
E) MgCl2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following elements are the least reactive?
A) alkali metals
B) noble gases
C) halogens
D) alkaline earth metals
E) metalloids
A) alkali metals
B) noble gases
C) halogens
D) alkaline earth metals
E) metalloids

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the empirical formula for hexane, C6H14?
A) C12H28
B) C6H14
C) C3H7
D) CH2.3
E) C0.43H
A) C12H28
B) C6H14
C) C3H7
D) CH2.3
E) C0.43H

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following ions occurs commonly?
A) P3+
B) Br7+
C) O6+
D) Ca2+
E) K-
A) P3+
B) Br7+
C) O6+
D) Ca2+
E) K-

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following groups does not contain any metals?
A) C, S, As, H
B) Cu, P, Se, Kr
C) N, Ne, Nd, Np
D) Xe, Hg, Ge, O
E) Cl, Al, Si, Ar
A) C, S, As, H
B) Cu, P, Se, Kr
C) N, Ne, Nd, Np
D) Xe, Hg, Ge, O
E) Cl, Al, Si, Ar

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
The colorless substance, MgF2, is used in the ceramics and glass industry. What is its name?
A) magnesium difluoride
B) magnesium fluoride
C) magnesium(II) fluoride
D) monomagnesium difluoride
E) none of the above, since they are all misspelled
A) magnesium difluoride
B) magnesium fluoride
C) magnesium(II) fluoride
D) monomagnesium difluoride
E) none of the above, since they are all misspelled

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds is covalent?
A) CaCl2
B) MgO
C) Al2O3
D) Cs2S
E) PCl3
A) CaCl2
B) MgO
C) Al2O3
D) Cs2S
E) PCl3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
Barium fluoride is used in embalming and in glass manufacturing. Which of the following gives the formula and bonding for barium fluoride?
A) BaF2, ionic compound
B) BaF2, covalent compound
C) BaF, ionic compound
D) BaF, covalent compound
E) Ba2F, ionic compound
A) BaF2, ionic compound
B) BaF2, covalent compound
C) BaF, ionic compound
D) BaF, covalent compound
E) Ba2F, ionic compound

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
Select the incorrect statement about elements and compounds.
A) All ionic compounds are neutral.
B) Some elements exist as molecules.
C) The bonding in compounds may be covalent or ionic.
D) The molecular formula of a compound provides more information than the structural formula.
E) Among the elements, there are more metals than non-metals.
A) All ionic compounds are neutral.
B) Some elements exist as molecules.
C) The bonding in compounds may be covalent or ionic.
D) The molecular formula of a compound provides more information than the structural formula.
E) Among the elements, there are more metals than non-metals.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
What is the chemical symbol for the group 6A (16) element that lies in period 4?
A) Cr
B) Hf
C) W
D) Ti
E) Se
A) Cr
B) Hf
C) W
D) Ti
E) Se

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
Which one of the following combinations of names and formulas of ions is incorrect?
A) O2- oxide
B) Cd2+ cadmium
C) ClO3- chlorate
D) HCO3- hydrogen carbonate
E) NO2- nitrate
A) O2- oxide
B) Cd2+ cadmium
C) ClO3- chlorate
D) HCO3- hydrogen carbonate
E) NO2- nitrate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
A red glaze on porcelain can be produced by using MnSO4. What is its name?
A) manganese disulfate
B) manganese(II) sulfate
C) manganese(IV) sulfate
D) manganese sulfate
E) manganese(I) sulfate
A) manganese disulfate
B) manganese(II) sulfate
C) manganese(IV) sulfate
D) manganese sulfate
E) manganese(I) sulfate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following is a polyatomic cation?
A) nitrate
B) chromate
C) permanganate
D) hydronium
E) potassium
A) nitrate
B) chromate
C) permanganate
D) hydronium
E) potassium

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following combinations of names and formulas of ions is incorrect?
A) NH4+ ammonium
B) S2- sulfide
C) CN- cyanide
D) S2O32- thiosulfate
E) ClO3- perchlorate
A) NH4+ ammonium
B) S2- sulfide
C) CN- cyanide
D) S2O32- thiosulfate
E) ClO3- perchlorate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
Sodium peroxide is an oxidizer used to bleach animal and vegetable fibers. What is its formula?
A) NaO
B) NaO2
C) Na2O2
D) Na2O
E) NaH2O2
A) NaO
B) NaO2
C) Na2O2
D) Na2O
E) NaH2O2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
Zinc acetate is used in preserving wood and in manufacturing glazes for porcelain. What is its formula?
A) ZnAc2
B) ZnCH3COO
C) Zn(CH3COO)2
D) Zn2CH3COO
E) ZnCH3COCH3
A) ZnAc2
B) ZnCH3COO
C) Zn(CH3COO)2
D) Zn2CH3COO
E) ZnCH3COCH3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
The compound, NaH2PO4, is present in many baking powders. What is its name?
A) sodium biphosphate
B) sodium hydrogen phosphate
C) sodium dihydrogen phosphate
D) sodium hydrophosphate
E) sodium dihydride phosphate
A) sodium biphosphate
B) sodium hydrogen phosphate
C) sodium dihydrogen phosphate
D) sodium hydrophosphate
E) sodium dihydride phosphate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
Barium sulfate is used in manufacturing photographic paper. What is its formula?
A) BaSO4
B) Ba(SO4)2
C) Ba2SO4
D) Ba2(SO4)3
E) BaSO3
A) BaSO4
B) Ba(SO4)2
C) Ba2SO4
D) Ba2(SO4)3
E) BaSO3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following combinations of names and formulas of ions is incorrect?
A) Ba2+ barium
B) S2- sulfate
C) CN- cyanide
D) ClO4- perchlorate
E) HCO3- bicarbonate
A) Ba2+ barium
B) S2- sulfate
C) CN- cyanide
D) ClO4- perchlorate
E) HCO3- bicarbonate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following combinations of names and formulas of ions is incorrect?
A) O2- oxide
B) Al3+ aluminum
C) NO3- nitrate
D) PO43- phosphate
E) CrO42- chromate
A) O2- oxide
B) Al3+ aluminum
C) NO3- nitrate
D) PO43- phosphate
E) CrO42- chromate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
What is the formula for lead (II) oxide?
A) PbO
B) PbO2
C) Pb2O
D) PbO4
E) Pb2O3
A) PbO
B) PbO2
C) Pb2O
D) PbO4
E) Pb2O3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
Ferric oxide is used as a pigment in metal polishing. Which of the following is its formula?
A) FeO
B) Fe2O
C) FeO3
D) Fe2O5
E) Fe2O3
A) FeO
B) Fe2O
C) FeO3
D) Fe2O5
E) Fe2O3

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
The substance, KClO3, is a strong oxidizer used in explosives, fireworks, and matches. What is its name?
A) potassium chlorite
B) potassium chloride
C) potassium(I) chlorite
D) potassium(I) chlorate
E) potassium chlorate
A) potassium chlorite
B) potassium chloride
C) potassium(I) chlorite
D) potassium(I) chlorate
E) potassium chlorate

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
The substance, CoCl2, is useful as a humidity indicator because it changes from pale blue to pink as it gains water from moist air. What is its name?
A) cobalt dichloride
B) cobalt(II) chloride
C) cobalt chloride
D) cobaltic chloride
E) copper(II) chloride
A) cobalt dichloride
B) cobalt(II) chloride
C) cobalt chloride
D) cobaltic chloride
E) copper(II) chloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
What is the name of Na2O?
A) disodium monoxide
B) sodium monoxide
C) sodium dioxide
D) sodium(I) oxide
E) sodium oxide
A) disodium monoxide
B) sodium monoxide
C) sodium dioxide
D) sodium(I) oxide
E) sodium oxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
In the ionic compound with the general formula M2X3, the likely charge on X is
A) +1.
B) +3.
C) -1.
D) -2.
E) -3.
A) +1.
B) +3.
C) -1.
D) -2.
E) -3.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
What is the formula for magnesium sulfide?
A) MgS
B) MgS2
C) Mg2S
D) Mg2S3
E) MgSO4
A) MgS
B) MgS2
C) Mg2S
D) Mg2S3
E) MgSO4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
Silver chloride is used in photographic emulsions. What is its formula?
A) Ag2Cl3
B) Ag2Cl
C) AgCl3
D) AgCl2
E) AgCl
A) Ag2Cl3
B) Ag2Cl
C) AgCl3
D) AgCl2
E) AgCl

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
The substance, CaSe, is used in materials which are electron emitters. What is its name?
A) calcium monoselenide
B) calcium(II) selenide
C) calcium selenide
D) calcium(I) selenide
E) calcium(II) selenium
A) calcium monoselenide
B) calcium(II) selenide
C) calcium selenide
D) calcium(I) selenide
E) calcium(II) selenium

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
The compound, (NH4)2S, can be used in analysis for trace amounts of metals present in a sample. What is its name?
A) ammonium sulfide
B) diammonium sulfide
C) ammonium sulfite
D) ammonia(I) sulfite
E) ammonium(I) sulfide
A) ammonium sulfide
B) diammonium sulfide
C) ammonium sulfite
D) ammonia(I) sulfite
E) ammonium(I) sulfide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
What is the name of PCl3?
A) phosphorus chloride
B) phosphoric chloride
C) phosphorus trichlorate
D) trichlorophosphide
E) phosphorus trichloride
A) phosphorus chloride
B) phosphoric chloride
C) phosphorus trichlorate
D) trichlorophosphide
E) phosphorus trichloride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
What is the name of the acid formed when HCN gas is dissolved in water?
A) cyanic acid
B) hydrocyanic acid
C) cyanous acid
D) hydrocyanous acid
E) hydrogen cyanide
A) cyanic acid
B) hydrocyanic acid
C) cyanous acid
D) hydrocyanous acid
E) hydrogen cyanide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the following combinations of names and formulas is incorrect?
A) H3PO4 phosphoric acid
B) HNO3 nitric acid
C) NaHCO3 sodium carbonate
D) H2CO3 carbonic acid
E) KOH potassium hydroxide
A) H3PO4 phosphoric acid
B) HNO3 nitric acid
C) NaHCO3 sodium carbonate
D) H2CO3 carbonic acid
E) KOH potassium hydroxide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
What is the name of P4Se3?
A) phosphorus selenide
B) phosphorus triselenide
C) tetraphosphorus selenide
D) phosphoric selenide
E) tetraphosphorus triselenide
A) phosphorus selenide
B) phosphorus triselenide
C) tetraphosphorus selenide
D) phosphoric selenide
E) tetraphosphorus triselenide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
Diiodine pentaoxide is used as an oxidizing agent that converts carbon monoxide to carbon dioxide. What is its chemical formula?
A) I2O5
B) IO5
C) 2IO5
D) I5O2
E) (IO5)2
A) I2O5
B) IO5
C) 2IO5
D) I5O2
E) (IO5)2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
Which one of the following formulas of ionic compounds is the least likely to be correct?
A) CaCl2
B) NaSO4
C) MgCO3
D) KF
E) Cu(NO3)2
A) CaCl2
B) NaSO4
C) MgCO3
D) KF
E) Cu(NO3)2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
The compound, P4S10, is used in the manufacture of safety matches. What is its name?
A) phosphorus sulfide
B) phosphoric sulfide
C) phosphorus decasulfide
D) tetraphosphorus decasulfide
E) phosphorus pentasulfide
A) phosphorus sulfide
B) phosphoric sulfide
C) phosphorus decasulfide
D) tetraphosphorus decasulfide
E) phosphorus pentasulfide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
What is the name of IF7?
A) iodine fluoride
B) iodic fluoride
C) iodine heptafluoride
D) heptafluoroiodide
E) heptafluorine iodide
A) iodine fluoride
B) iodic fluoride
C) iodine heptafluoride
D) heptafluoroiodide
E) heptafluorine iodide

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of the acid formed when H2S gas is dissolved in water?
A) sulfuric acid
B) sulfurous acid
C) hydrosulfuric acid
D) hydrosulfurous acid
E) sulfidic acid
A) sulfuric acid
B) sulfurous acid
C) hydrosulfuric acid
D) hydrosulfurous acid
E) sulfidic acid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
Calcium hydroxide is used in mortar, plaster, and cement. What is its formula?
A) CaOH
B) CaOH2
C) Ca2OH
D) Ca(OH)2
E) CaHO2
A) CaOH
B) CaOH2
C) Ca2OH
D) Ca(OH)2
E) CaHO2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
Iron (III) chloride hexahydrate is used as a coagulant for sewage and industrial wastes. What is its formula?
A) Fe(Cl6H2O)3
B) Fe3Cl6H2O
C) FeCl3(H2O)6
D) Fe3Cl(H2O)6
E) FeCl36H2O
A) Fe(Cl6H2O)3
B) Fe3Cl6H2O
C) FeCl3(H2O)6
D) Fe3Cl(H2O)6
E) FeCl36H2O

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
What is the formula for lithium nitrite?
A) LiNO2
B) Li2NO2
C) LiNO3
D) Li2NO3
E) LiNO4
A) LiNO2
B) Li2NO2
C) LiNO3
D) Li2NO3
E) LiNO4

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
What is the name of the acid formed when HClO4 liquid is dissolved in water?
A) hydrochloric acid
B) perchloric acid
C) chloric acid
D) chlorous acid
E) hydrochlorate acid
A) hydrochloric acid
B) perchloric acid
C) chloric acid
D) chlorous acid
E) hydrochlorate acid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
What is the name of the acid formed when HBr gas is dissolved in water?
A) bromic acid
B) bromous acid
C) hydrobromic acid
D) hydrobromous acid
E) hydrobromidic acid
A) bromic acid
B) bromous acid
C) hydrobromic acid
D) hydrobromous acid
E) hydrobromidic acid

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
Tetrasulfur dinitride decomposes explosively when heated. What is its formula?
A) S2N4
B) S4N2
C) 4SN2
D) S4N
E) S2N
A) S2N4
B) S4N2
C) 4SN2
D) S4N
E) S2N

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
Chlorine dioxide is a strong oxidizer that is used for bleaching flour and textiles and for purification of water. What is its formula?
A) (ClO)2
B) Cl2O
C) Cl2O2
D) Cl2O4
E) ClO2
A) (ClO)2
B) Cl2O
C) Cl2O2
D) Cl2O4
E) ClO2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
What is the name of BBr3?
A) boron bromide
B) boric bromide
C) boron tribromide
D) tribromoboride
E) bromine triboride
A) boron bromide
B) boric bromide
C) boron tribromide
D) tribromoboride
E) bromine triboride

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of the following formulas of ionic compounds is the least likely to be correct?
A) NH4Cl
B) Ba(OH)2
C) Na2SO4
D) Ca2NO3
E) Cu(CN)2
A) NH4Cl
B) Ba(OH)2
C) Na2SO4
D) Ca2NO3
E) Cu(CN)2

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
Potassium permanganate is a strong oxidizer that reacts explosively with easily oxidized materials. What is its formula?
A) KMnO3
B) KMnO4
C) K2MnO4
D) K(MnO4)2
E) K2Mn2O7
A) KMnO3
B) KMnO4
C) K2MnO4
D) K(MnO4)2
E) K2Mn2O7

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
The name for HF(g) is
A) hydrofluoric acid.
B) hydrogen(I) fluoride.
C) hydrogen fluoride.
D) hydrogen fluorine.
E) fluoric acid.
A) hydrofluoric acid.
B) hydrogen(I) fluoride.
C) hydrogen fluoride.
D) hydrogen fluorine.
E) fluoric acid.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


