Deck 5: Gases and the Kinetic-Molecular Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 5: Gases and the Kinetic-Molecular Theory
1
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its Celsius temperature, other factors remaining constant? 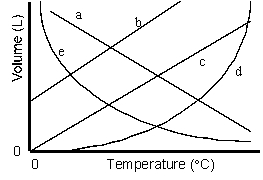

B
2
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its pressure, other factors remaining constant? 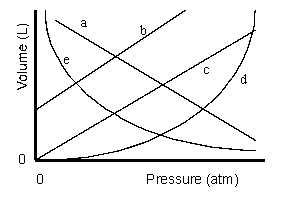

e
3
"The volume of an ideal gas is directly proportional to its absolute temperature at constant pressure and number of moles" is a statement of ________________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Dalton's
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Dalton's
Charles's
4
A flask containing neon gas is connected to an open-ended mercury manometer. The open end is exposed to the atmosphere, where the prevailing pressure is 745 torr. The mercury level in the open arm is 50. mm below that in the arm connected to the flask of neon. What is the neon pressure, in torr?
A) -50. torr
B) 50. torr
C) 695 torr
D) 795 torr
E) none of the above
A) -50. torr
B) 50. torr
C) 695 torr
D) 795 torr
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
"The volume of an ideal gas is directly proportional to the number of moles of the gas at constant temperature and pressure" is a statement of _____________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Dalton's
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Dalton's

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
A ballerina weighs 103 lbs and is up on her toes with only 10.0 cm2 of her slippers in contact with the floor. What pressure is she exerting on the floor?
A) 4.59 × 105 Pa
B) 4.59 × 103 Pa
C) 4.59 × 101 Pa
D) 4.59 × 10-1 Pa
E) 4.59 × 10-3 Pa
A) 4.59 × 105 Pa
B) 4.59 × 103 Pa
C) 4.59 × 101 Pa
D) 4.59 × 10-1 Pa
E) 4.59 × 10-3 Pa

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
The pressure of hydrogen sulfide gas in a container is 35,650 Pa. What is this pressure in torr?
A) 46.91 torr
B) 267.4 torr
C) 351.8 torr
D) 3612 torr
E) 27090 torr
A) 46.91 torr
B) 267.4 torr
C) 351.8 torr
D) 3612 torr
E) 27090 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
"The pressure of an ideal gas is inversely proportional to its volume at constant temperature and number of moles" is a statement of __________________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its absolute temperature, other factors remaining constant? 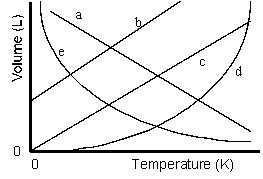


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
A flask containing argon gas is connected to a closed-ended mercury manometer. The closed end is under vacuum. If the mercury level in the closed arm is 230. mm above that in the arm connected to the flask, what is the argon pressure, in torr?
A) -230.
B) 230.
C) 530.
D) 790.
E) none of the above
A) -230.
B) 230.
C) 530.
D) 790.
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
The air pressure in a volleyball is 75 psi. What is this pressure in torr?
A) 520 torr
B) 562 torr
C) 3900 torr
D) 7600 torr
E) 75000 torr
A) 520 torr
B) 562 torr
C) 3900 torr
D) 7600 torr
E) 75000 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
Hydrogen gas exerts a pressure of 466 torr in a container. What is this pressure in atmospheres?
A) 0.217 atm
B) 0.466 atm
C) 0.613 atm
D) 1.63 atm
E) 4.60 atm
A) 0.217 atm
B) 0.466 atm
C) 0.613 atm
D) 1.63 atm
E) 4.60 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
"The pressure of an ideal gas is directly proportional to its absolute temperature at constant volume and number of moles" is a statement of ________________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
A flask containing helium gas is connected to an open-ended mercury manometer. The open end is exposed to the atmosphere, where the prevailing pressure is 752 torr. The mercury level in the open arm is 26 mm above that in the arm connected to the flask of helium. What is the helium pressure, in torr?
A) -26 torr
B) 26 torr
C) 726 torr
D) 778 torr
E) none of the above
A) -26 torr
B) 26 torr
C) 726 torr
D) 778 torr
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
"The rate of effusion of a gas is inversely proportional to the square root of its molar mass" is a statement of ______________________ Law.
A) Charles's
B) Graham's
C) Dalton's
D) Avogadro's
E) Boyle's
A) Charles's
B) Graham's
C) Dalton's
D) Avogadro's
E) Boyle's

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
"The total pressure in a mixture of unreacting gases is equal to the sum of the partial pressures of the individual gases" is a statement of __________________ Law.
A) Charles's
B) Graham's
C) Boyle's
D) Avogadro's
E) Dalton's
A) Charles's
B) Graham's
C) Boyle's
D) Avogadro's
E) Dalton's

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
The pressure of sulfur dioxide in a container is 159 kPa. What is this pressure in atmospheres?
A) 0.209 atm
B) 0.637 atm
C) 1.57 atm
D) 21.2 atm
E) 15900 atm
A) 0.209 atm
B) 0.637 atm
C) 1.57 atm
D) 21.2 atm
E) 15900 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Given that pressure has dimensions of force ÷ area; that force has dimensions of mass × acceleration; and that the S.I. unit of pressure is the pascal, what is 1 pascal in terms of S.I. base units?
A) 1 Pa = 1000 g/cms2
B) 1 Pa = 1 g/ms2
C) 1 Pa = 10-3 kgm/s2
D) 1 Pa = 1 kgm/s2
E) 1 Pa = 1 kg/ms2
A) 1 Pa = 1000 g/cms2
B) 1 Pa = 1 g/ms2
C) 1 Pa = 10-3 kgm/s2
D) 1 Pa = 1 kgm/s2
E) 1 Pa = 1 kg/ms2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
Mercury is 13.6 times as dense as liquid water. What would be the reading of a water-filled barometer at normal atmospheric pressure, 760. mmHg?
A) 1.03 × 103 torr
B) 1.03 × 103 Pa
C) 1.03 × 103 mm height of water column
D) 1.03 × 103 cm height of water column
E) 13.6 atm
A) 1.03 × 103 torr
B) 1.03 × 103 Pa
C) 1.03 × 103 mm height of water column
D) 1.03 × 103 cm height of water column
E) 13.6 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
Mineral oil can be used in place of mercury in manometers when small pressure changes are to be measured. What is the pressure of an oxygen sample in mm of mineral oil if its pressure is 28.5 mm Hg? (d of mineral oil = 0.88 g/mL; d of Hg = 13.5 g/mL)
A) 1.9 mm mineral oil
B) 15 mm mineral oil
C) 32 mm mineral oil
D) 380 mm mineral oil
E) 440 mm mineral oil
A) 1.9 mm mineral oil
B) 15 mm mineral oil
C) 32 mm mineral oil
D) 380 mm mineral oil
E) 440 mm mineral oil

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
A sample of ammonia gas at 65.5°C and 524 torr has a volume of 15.31 L. What is its volume when the temperature is -15.8°C and its pressure is 524 torr?
A) 3.69 L
B) 11.6 L
C) 20.2 L
D) 63.5 L
E) not possible, since the volume would have to be negative
A) 3.69 L
B) 11.6 L
C) 20.2 L
D) 63.5 L
E) not possible, since the volume would have to be negative

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the lines on the figure below is the best representation of the relationship between the volume and the number of moles of a gas, measured at constant temperature and pressure? 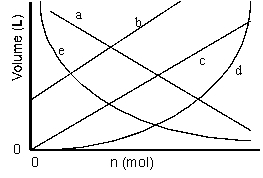


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
A sample container of carbon monoxide occupies a volume of 435 mL at a pressure of 785 torr and a temperature of 298 K. What would its temperature be if the volume were changed to 265 mL at a pressure of 785 torr?
A) 182 K
B) 298 K
C) 387 K
D) 489 K
E) 538 K
A) 182 K
B) 298 K
C) 387 K
D) 489 K
E) 538 K

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of propane, a component of LP gas, has a volume of 35.3 L at 315 K and 922 torr. What is its volume at STP?
A) 25.2 L
B) 30.6 L
C) 33.6 L
D) 37.1 L
E) 49.2 L
A) 25.2 L
B) 30.6 L
C) 33.6 L
D) 37.1 L
E) 49.2 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
A sample of nitrogen gas at 298 K and 745 torr has a volume of 37.42 L. What volume will it occupy if the pressure is increased to 894 torr at constant temperature?
A) 22.3 L
B) 31.2 L
C) 44.9 L
D) 112 L
E) 380 L
A) 22.3 L
B) 31.2 L
C) 44.9 L
D) 112 L
E) 380 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
A sample of the inert gas krypton has its pressure tripled while its temperature remained constant. If the original volume is 12 L, what is the final volume?
A) 4.0 L
B) 6.0 L
C) 9 L
D) 36 L
E) 48 L
A) 4.0 L
B) 6.0 L
C) 9 L
D) 36 L
E) 48 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of methane gas, CH4(g), occupies a volume of 60.3 L at a pressure of 469 torr and a temperature of 29.3°C. What would be its temperature at a pressure of 243 torr and volume of 60.3 L?
A) -116.5°C
B) 15.2°C
C) 15.5°C
D) 57.7°C
E) 310.6°C
A) -116.5°C
B) 15.2°C
C) 15.5°C
D) 57.7°C
E) 310.6°C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
Nitrogen dioxide is a red-brown gas that is responsible for the color of photochemical smog. A sample of nitrogen dioxide has a volume of 28.6 L at 45.3°C and 89.9 kPa. What is its volume at STP?
A) 21.8 L
B) 27.6 L
C) 29.6 L
D) 37.6 L
E) 153 L
A) 21.8 L
B) 27.6 L
C) 29.6 L
D) 37.6 L
E) 153 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
A carbon dioxide sample weighing 44.0 g occupies 32.68 L at 65°C and 645 torr. What is its volume at STP?
A) 22.4 L
B) 31.1 L
C) 34.3 L
D) 35.2 L
E) 47.7 L
A) 22.4 L
B) 31.1 L
C) 34.3 L
D) 35.2 L
E) 47.7 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
A 500-mL sample of argon at 800 torr has its absolute temperature quadrupled. If the volume remains unchanged what is the new pressure?
A) 200 torr
B) 400 torr
C) 800 torr
D) 2400 torr
E) 3200 torr
A) 200 torr
B) 400 torr
C) 800 torr
D) 2400 torr
E) 3200 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
What are the conditions of STP?
A) 0 K and l atm
B) 273.15 K and 760 torr
C) 0°C and 760 atm
D) 273.15°C and 760 torr
E) none of the above
A) 0 K and l atm
B) 273.15 K and 760 torr
C) 0°C and 760 atm
D) 273.15°C and 760 torr
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
A sample of an ideal gas has its volume doubled while its temperature remains constant. If the original pressure was 100 torr, what is the new pressure?
A) 10 torr
B) 50 torr
C) 100 torr
D) 200 torr
E) 1000 torr
A) 10 torr
B) 50 torr
C) 100 torr
D) 200 torr
E) 1000 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the pressure of a helium sample at -207.3°C and 768 mL if it exerts a pressure of 175 kPa at 25.0°C and 925 mL.
A) 32.1 kPa
B) 46.6 kPa
C) 657 kPa
D) 953 kPa
E) not possible, since the pressure would have to be negative
A) 32.1 kPa
B) 46.6 kPa
C) 657 kPa
D) 953 kPa
E) not possible, since the pressure would have to be negative

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
A sample of nitrogen gas is confined to a 14.0 L container at 375 torr and 37.0°C. How many moles of nitrogen are in the container?
A) 0.271 mol
B) 2.27 mol
C) 3.69 mol
D) 206 mol
E) 227 mol
A) 0.271 mol
B) 2.27 mol
C) 3.69 mol
D) 206 mol
E) 227 mol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
A sample of oxygen gas has its absolute temperature halved while the pressure of the gas remained constant. If the initial volume is 400 mL, what is the final volume?
A) 20 mL
B) 133 mL
C) 200 mL
D) 400 mL
E) 800 mL
A) 20 mL
B) 133 mL
C) 200 mL
D) 400 mL
E) 800 mL

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
A weather balloon was initially at a pressure of 0.950 atm, and its volume was 35.0 L. The pressure decreased to 0.750 atm, without loss of gas or change in temperature. What was the change in the volume?
A) increased by 44.3 L
B) increased by 9.3 L
C) increased by 7.4 L
D) decreased by 27.6 L
E) decreased by 7.4 L
A) increased by 44.3 L
B) increased by 9.3 L
C) increased by 7.4 L
D) decreased by 27.6 L
E) decreased by 7.4 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
A 0.850-mole sample of nitrous oxide, a gas used as an anesthetic by dentists, has a volume of 20.46 L at 123°C and 1.35 atm. What would be its volume at 468°C and 1.35 atm?
A) 5.38 L
B) 10.9 L
C) 19.0 L
D) 38.3 L
E) 77.9 L
A) 5.38 L
B) 10.9 L
C) 19.0 L
D) 38.3 L
E) 77.9 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the temperature of an argon sample at 55.4 kPa and 18.6 L if it occupies 25.8 L at 75.0°C and 41.1 kPa.
A) 95.0°C
B) 85.1°C
C) 77.2°C
D) 72.9°C
E) 65.2°C
A) 95.0°C
B) 85.1°C
C) 77.2°C
D) 72.9°C
E) 65.2°C

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
A 750-mL sample of hydrogen exerts a pressure of 822 torr at 325 K. What pressure does it exert if the temperature is raised to 475 K at constant volume?
A) 188 torr
B) 562 torr
C) 1.11 × 103 torr
D) 1.20 × 103 torr
E) 1.90 × 103 torr
A) 188 torr
B) 562 torr
C) 1.11 × 103 torr
D) 1.20 × 103 torr
E) 1.90 × 103 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L. What will the gas pressure be if the volume is increased to 321 L at 125°C?
A) 212 torr
B) 289 torr
C) 356 torr
D) 441 torr
E) 359 torr
A) 212 torr
B) 289 torr
C) 356 torr
D) 441 torr
E) 359 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
Hydrochloric acid is prepared by bubbling hydrogen chloride gas through water. What is the concentration of a solution prepared by dissolving 225 L of HCl(g) at 37°C and 89.6 kPa in 5.25 L of water?
A) 1.49 M
B) 1.66 M
C) 7.82 M
D) 12.5 M
E) 16.6 M
A) 1.49 M
B) 1.66 M
C) 7.82 M
D) 12.5 M
E) 16.6 M

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
Assuming ideal behavior, what is the density of argon gas at STP, in g/L?
A) 0.0176 g/L
B) 0.0250 g/L
C) 0.0561 g/L
D) 1.78 g/L
E) 181. g/L
A) 0.0176 g/L
B) 0.0250 g/L
C) 0.0561 g/L
D) 1.78 g/L
E) 181. g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
Ima Chemist found the density of Freon-11 (CFCl3) to be 5.58 g/L under her experimental conditions. Her measurements showed that the density of an unknown gas was 4.38 g/L under the same conditions. What is the molar mass of the unknown?
A) 96.7 g/mol
B) 108 g/mol
C) 127 g/mol
D) 165 g/mol
E) 175 g/mol
A) 96.7 g/mol
B) 108 g/mol
C) 127 g/mol
D) 165 g/mol
E) 175 g/mol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
What is the pressure in a 7.50-L flask if 0.15 mol of carbon dioxide is added to 0.33 mol of oxygen? The temperature of the mixture is 48.0°C.
A) 0.252 atm
B) 0.592 atm
C) 1.69 atm
D) 3.96 atm
E) 4.80 atm
A) 0.252 atm
B) 0.592 atm
C) 1.69 atm
D) 3.96 atm
E) 4.80 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
A gas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). If the partial pressure of argon is 200. torr, what is the pressure of methane, in torr?
A) 80.0 torr
B) 200. torr
C) 256 torr
D) 500. torr
E) 556 torr
A) 80.0 torr
B) 200. torr
C) 256 torr
D) 500. torr
E) 556 torr

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
A flask with a volume of 3.16 L contains 9.33 grams of an unknown gas at 32.0°C and 1.00 atm. What is the molar mass of the gas?
A) 7.76 g/mol
B) 66.1 g/mol
C) 74.0 g/mol
D) 81.4 g/mol
E) 144 g/mol
A) 7.76 g/mol
B) 66.1 g/mol
C) 74.0 g/mol
D) 81.4 g/mol
E) 144 g/mol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
Dr. I. M. A. Brightguy adds 0.1727 g of an unknown gas to a 125-mL flask. If Dr. B finds the pressure to be 736 torr at 20.0°C, is the gas likely to be methane, CH4, nitrogen, N2, oxygen, O2, neon, Ne, or argon, Ar?
A) CH4
B) N2
C) Ne
D) Ar
E) O2
A) CH4
B) N2
C) Ne
D) Ar
E) O2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
Linolenic acid (C18H30O2) can be hydrogenated to stearic acid by reacting it with hydrogen gas according to the equation: C18H30O2 + 3H2 C18H36O2
What volume of hydrogen gas, measured at STP, is required to react with 10.5 g of linolenic acid in this reaction?
A) 2.53 L
B) 1.69 L
C) 1.27 L
D) 845 mL
E) 422 mL
What volume of hydrogen gas, measured at STP, is required to react with 10.5 g of linolenic acid in this reaction?
A) 2.53 L
B) 1.69 L
C) 1.27 L
D) 845 mL
E) 422 mL

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
Hydrogen peroxide was catalytically decomposed and 75.3 mL of oxygen gas was collected over water at 25°C and 742 torr. What mass of oxygen was collected? (Pwater = 24 torr at 25°C)
A) 0.00291 g
B) 0.0931 g
C) 0.0962 g
D) 0.0993 g
E) 0.962 g
A) 0.00291 g
B) 0.0931 g
C) 0.0962 g
D) 0.0993 g
E) 0.962 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
Magnesium metal (0.100 mol) and a volume of aqueous hydrochloric acid that contains 0.500 mol of HCl are combined and react to completion. How many liters of hydrogen gas, measured at STP, are produced? Mg(s) + 2HCl(aq) MgCl2(aq) + H2(g)
A) 2.24 L of H2
B) 4.48 L of H2
C) 5.60 L of H2
D) 11.2 L of H2
E) 22.4 L of H2
A) 2.24 L of H2
B) 4.48 L of H2
C) 5.60 L of H2
D) 11.2 L of H2
E) 22.4 L of H2

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
An unknown liquid is vaporized in a 273-mL flask by immersion in a water bath at 99°C. The barometric pressure is 753 torr. If the mass of the vapor retained in the flask is 1.362 g, what is its molar mass?
A) 20.4 g/mol
B) 40.9 g/mol
C) 112 g/mol
D) 154 g/mol
E) 184 g/mol
A) 20.4 g/mol
B) 40.9 g/mol
C) 112 g/mol
D) 154 g/mol
E) 184 g/mol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
If 0.750 L of argon at 1.50 atm and 177°C and 0.235 L of sulfur dioxide at 95.0 kPa and 63.0°C are added to a 1.00-L flask and the flask's temperature is adjusted to 25.0°C, what is the resulting pressure in the flask?
A) 0.0851 atm
B) 0.244 atm
C) 0.946 atm
D) 1.74 atm
E) 1.86 atm
A) 0.0851 atm
B) 0.244 atm
C) 0.946 atm
D) 1.74 atm
E) 1.86 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
Lithium oxide is an effective absorber of carbon dioxide and can be used to purify air in confined areas such as space vehicles. What volume of carbon dioxide can be absorbed by 1.00 kg of lithium oxide at 25°C and 1.00 atm? Li2O(aq) + CO2(g) Li2CO3(s)
A) 687 mL
B) 819 mL
C) 687 L
D) 819 L
E) 22.4 L
A) 687 mL
B) 819 mL
C) 687 L
D) 819 L
E) 22.4 L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
A compressed gas cylinder containing 1.50 mol methane has a volume of 3.30 L. What pressure does the methane exert on the walls of the cylinder if its temperature is 25°C?
A) 9.00 × 10-2 atm
B) 0.933 atm
C) 1.11 atm
D) 1.70 atm
E) 11.1 atm
A) 9.00 × 10-2 atm
B) 0.933 atm
C) 1.11 atm
D) 1.70 atm
E) 11.1 atm

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Small quantities of hydrogen can be prepared by the addition of hydrochloric acid to zinc. A sample of 195 mL of hydrogen was collected over water at 25°C and 753 torr. What mass of hydrogen was collected? (Pwater = 24 torr at 25°C)
A) 0.00765 g
B) 0.0154 g
C) 0.0159 g
D) 0.0164 g
E) 0.159 g
A) 0.00765 g
B) 0.0154 g
C) 0.0159 g
D) 0.0164 g
E) 0.159 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
What is the density of carbon dioxide gas at -25.2°C and 98.0 kPa?
A) 0.232 g/L
B) 0.279 g/L
C) 0.994 g/L
D) 1.74 g/L
E) 2.09 g/L
A) 0.232 g/L
B) 0.279 g/L
C) 0.994 g/L
D) 1.74 g/L
E) 2.09 g/L

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
A gas consists of 85.7 % carbon and 14.3 % hydrogen, by weight. A sample of this gas weighing 0.673 g occupies 729 mL at a pressure of 720.0 mmHg and a temperature of 77°C. Calculate its empirical and molecular formulas.
A) CH, C2H2
B) CH2, C2H4
C) CH2, C3H6
D) CH3, C2H6
E) CH4, CH4
A) CH, C2H2
B) CH2, C2H4
C) CH2, C3H6
D) CH3, C2H6
E) CH4, CH4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
A gas mixture, with a total pressure of 300. torr, consists of equal masses of Ne (atomic weight 20.) and Ar (atomic weight 40.). What is the partial pressure of Ar, in torr?
A) 75 torr
B) 100. torr
C) 150. torr
D) 200. torr
E) none of the above
A) 75 torr
B) 100. torr
C) 150. torr
D) 200. torr
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
Methane, CH4(g), reacts with steam to give synthesis gas, a mixture of carbon monoxide and hydrogen, which is used as starting material for the synthesis of a number of organic and inorganic compounds. CH4(g) + H2O(g) CO(g) + H2(g) [unbalanced]
What mass of hydrogen is formed if 275 L of methane (measured at STP) is converted to synthesis gas?
A) 12.3 g
B) 24.7 g
C) 37.1 g
D) 49.4 g
E) 74.2 g
What mass of hydrogen is formed if 275 L of methane (measured at STP) is converted to synthesis gas?
A) 12.3 g
B) 24.7 g
C) 37.1 g
D) 49.4 g
E) 74.2 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
A 250.0-mL sample of ammonia, NH3(g), exerts a pressure of 833 torr at 42.4°C. What mass of ammonia is in the container?
A) 0.0787 g
B) 0.180 g
C) 8.04 g
D) 17.0 g
E) 59.8 g
A) 0.0787 g
B) 0.180 g
C) 8.04 g
D) 17.0 g
E) 59.8 g

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
The ozone layer is important because
A) ozone absorbs low energy radiation which warms the troposphere.
B) ozone purifies the atmosphere by reacting with excess fluorocarbons.
C) ozone absorbs ultraviolet radiation.
D) ozone reflects high energy radiation such as X-rays and gamma rays.
E) humans need to breathe air containing some ozone.
A) ozone absorbs low energy radiation which warms the troposphere.
B) ozone purifies the atmosphere by reacting with excess fluorocarbons.
C) ozone absorbs ultraviolet radiation.
D) ozone reflects high energy radiation such as X-rays and gamma rays.
E) humans need to breathe air containing some ozone.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
A 3.0-L sample of helium was placed in a container fitted with a porous membrane. Half of the helium effused through the membrane in 24 hours. A 3.0-L sample of oxygen was placed in an identical container. How many hours will it take for half of the oxygen to effuse through the membrane?
A) 8.5 h
B) 12 h
C) 48 h
D) 60. h
E) 68 h
A) 8.5 h
B) 12 h
C) 48 h
D) 60. h
E) 68 h

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following gases will be the slowest to diffuse through a room?
A) methane, CH4
B) hydrogen sulfide, H2S
C) carbon dioxide, CO2
D) water, H2O
E) neon, Ne
A) methane, CH4
B) hydrogen sulfide, H2S
C) carbon dioxide, CO2
D) water, H2O
E) neon, Ne

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
Select the gas with the highest average kinetic energy per mole at 298 K.
A) O2
B) CO2
C) H2O
D) H2
E) All have the same average kinetic energy.
A) O2
B) CO2
C) H2O
D) H2
E) All have the same average kinetic energy.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following gases effuses most rapidly?
A) nitrogen
B) oxygen
C) hydrogen chloride
D) ammonia
E) carbon monoxide
A) nitrogen
B) oxygen
C) hydrogen chloride
D) ammonia
E) carbon monoxide

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
The temperature of the carbon dioxide atmosphere near the surface of Venus is 475°C. Calculate the average kinetic energy per mole of carbon dioxide molecules on Venus.
A) 2520 J/mol
B) 4150 J/mol
C) 5920 J/mol
D) 9330 J/mol
E) 5920 kJ/mol
A) 2520 J/mol
B) 4150 J/mol
C) 5920 J/mol
D) 9330 J/mol
E) 5920 kJ/mol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
Arrange the following gases in order of increasing rate of effusion. C2H6 Ar HCl PH3
A) Ar < HCl < PH3 < C2H6
B) C2H6 < PH3 < HCl < Ar
C) Ar < PH3 < C2H6 < HCl
D) C2H6 < HCl < PH3 < Ar
E) Ar < PH3 < HCl < C2H6
A) Ar < HCl < PH3 < C2H6
B) C2H6 < PH3 < HCl < Ar
C) Ar < PH3 < C2H6 < HCl
D) C2H6 < HCl < PH3 < Ar
E) Ar < PH3 < HCl < C2H6

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
Select the gas with the largest root-mean-square molecular speed at 25°C.
A) NH3
B) CO
C) H2
D) SF6
E) All the gases have the same root-mean-square molecular speed at 25°C.
A) NH3
B) CO
C) H2
D) SF6
E) All the gases have the same root-mean-square molecular speed at 25°C.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
The most probable speed of an oxygen molecule in the gas phase at room temperature is 450 m/s. The root-mean-square speed (urms) is therefore
A) equal to 450 m/s.
B) slightly less than 450 m/s.
C) much less than 450 m/s.
D) slightly greater than 450 m/s.
E) much greater than 450 m/s.
A) equal to 450 m/s.
B) slightly less than 450 m/s.
C) much less than 450 m/s.
D) slightly greater than 450 m/s.
E) much greater than 450 m/s.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
At what temperature in kelvin is the root mean square speed of helium atoms (atomic weight = 4.00) equal to that of oxygen molecules (molecular weight = 32.00) at 300. K?
A) 37.5 K
B) 75 K
C) 106 K
D) 292 K
E) 2400. K
A) 37.5 K
B) 75 K
C) 106 K
D) 292 K
E) 2400. K

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
Calculate the root-mean-square speed of methane, CH4 (g), at 78°C.
A) 23 m/s
B) 350 m/s
C) 550 m/s
D) 667 m/s
E) 740 m/s
A) 23 m/s
B) 350 m/s
C) 550 m/s
D) 667 m/s
E) 740 m/s

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
Calculate the rms speed of carbon dioxide molecules at STP.
A) 12.4 m/s
B) 155 m/s
C) 393 m/s
D) 1.55 × 105 m/s
E) The answer can't be calculated without more data.
A) 12.4 m/s
B) 155 m/s
C) 393 m/s
D) 1.55 × 105 m/s
E) The answer can't be calculated without more data.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
Freon-12, CF2Cl2, which has been widely used in air conditioning systems, is considered a threat to the ozone layer in the stratosphere. Calculate the root-mean-square velocity of Freon-12 molecules in the lower stratosphere where the temperature is -65°C.
A) 20 m/s
B) 120 m/s
C) 210 m/s
D) 260 m/s
E) 4.4 × 104 m/s
A) 20 m/s
B) 120 m/s
C) 210 m/s
D) 260 m/s
E) 4.4 × 104 m/s

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
A compound composed of carbon, hydrogen, and chlorine effuses through a pinhole 0.411 times as fast as neon. Select the correct molecular formula for the compound.
A) CHCl3
B) CH2Cl2
C) C2H2Cl2
D) C2H3Cl
E) CCl4
A) CHCl3
B) CH2Cl2
C) C2H2Cl2
D) C2H3Cl
E) CCl4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
Helium gas is being pumped into a rigid container at a constant temperature. As a result, the pressure of helium in the container is increasing. Select the one correct statement below.
A) As the pressure increases, helium atoms move faster, on average.
B) As the pressure increases, helium atoms move more slowly, on average.
C) As the pressure increases, the volume of the container must decrease.
D) As the pressure increases, helium atoms stay closer to the wall of the container, on average.
E) As the pressure increases, there are more collisions of helium atoms with the container wall.
A) As the pressure increases, helium atoms move faster, on average.
B) As the pressure increases, helium atoms move more slowly, on average.
C) As the pressure increases, the volume of the container must decrease.
D) As the pressure increases, helium atoms stay closer to the wall of the container, on average.
E) As the pressure increases, there are more collisions of helium atoms with the container wall.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
Select the statement which does NOT apply to an ideal gas.
A) There are no attractive forces between the gas molecules.
B) There are strong repulsive forces between the gas molecules.
C) The volume occupied by the molecules is negligible compared to the container volume.
D) The gas behaves according to the ideal gas equation.
E) The average kinetic energy of the molecules is proportional to the absolute temperature.
A) There are no attractive forces between the gas molecules.
B) There are strong repulsive forces between the gas molecules.
C) The volume occupied by the molecules is negligible compared to the container volume.
D) The gas behaves according to the ideal gas equation.
E) The average kinetic energy of the molecules is proportional to the absolute temperature.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
Nitrogen will behave most like an ideal gas
A) at high temperature and high pressure.
B) at high temperature and low pressure.
C) at low temperature and high pressure.
D) at low temperature and low pressure.
E) at intermediate (moderate) temperature and pressure.
A) at high temperature and high pressure.
B) at high temperature and low pressure.
C) at low temperature and high pressure.
D) at low temperature and low pressure.
E) at intermediate (moderate) temperature and pressure.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following changes will NOT affect the total pressure of gas in a container, assuming all other factors remain constant?
A) The frequency of collisions of molecules with the walls is increased.
B) The average velocity of the molecules is lowered.
C) The temperature of the sample is altered.
D) Half of the molecules are replaced by an equal number of molecules of a gas with a different molecular weight.
E) The total number of molecules is altered.
A) The frequency of collisions of molecules with the walls is increased.
B) The average velocity of the molecules is lowered.
C) The temperature of the sample is altered.
D) Half of the molecules are replaced by an equal number of molecules of a gas with a different molecular weight.
E) The total number of molecules is altered.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
If the molecular mass of a gas increases by a factor of 4 at constant temperature, its rms speed will
A) decrease by a factor of 4.
B) increase by a factor of 4.
C) decrease by a factor of 16.
D) increase by a factor of 16.
E) decrease by a factor of 2.
A) decrease by a factor of 4.
B) increase by a factor of 4.
C) decrease by a factor of 16.
D) increase by a factor of 16.
E) decrease by a factor of 2.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
The volume of a single molecule of water is 2.99 × 10-23 mL. For a sample of gaseous water at 1.00 atm and 150 °C, what fraction of the container's volume is occupied by the molecules themselves?
A) 5.2 × 10-7
B) 4.5 × 10-5
C) 5.2 × 10-4
D) 5.2 × 10-1
E) none of the above
A) 5.2 × 10-7
B) 4.5 × 10-5
C) 5.2 × 10-4
D) 5.2 × 10-1
E) none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck


