Deck 4: Alkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/56
Play
Full screen (f)
Deck 4: Alkanes
1
Which of the following compounds has primary, secondary and tertiary hydrogen atoms?
A) Pentane
B) Hexane
C) 2-Methylpentane
D) 2,2-Dimethylpentane
A) Pentane
B) Hexane
C) 2-Methylpentane
D) 2,2-Dimethylpentane
2-Methylpentane
2
How many constitutional isomers are there with the molecular formula C5H12?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5
3
3
What is the parent chain for the following compound? 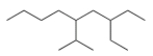
A) Heptane
B) Octane
C) Nonane
D) Decane

A) Heptane
B) Octane
C) Nonane
D) Decane
Nonane
4
What is the IUPAC name for the following compound? 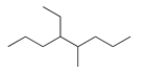
A) 4-Ethyl-5-methyloctane
B) 4-Methyl-5-ethyloctane
C) 4-Methyl-3-propylheptane
D) 4-Methyl-5-propyloctane

A) 4-Ethyl-5-methyloctane
B) 4-Methyl-5-ethyloctane
C) 4-Methyl-3-propylheptane
D) 4-Methyl-5-propyloctane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds has only primary, secondary and quaternary carbon atoms?
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
6
How many constitutional isomers are there with the molecular formula C6H14?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds has primary, secondary, tertiary and quaternary carbon atoms?
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
8
What is the parent chain for the following compound? 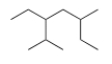
A) Hexane
B) Heptane
C) Octane
D) Nonane

A) Hexane
B) Heptane
C) Octane
D) Nonane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements about alkanes is not true?
A) Alkanes are aliphatic hydrocarbons.
B) Alkanes contain only C-C and C-H bonds.
C) Alkanes are acyclic or cyclic.
D) Acyclic alkanes have two fewer H atoms than cyclic alkanes with the same number of carbons.
A) Alkanes are aliphatic hydrocarbons.
B) Alkanes contain only C-C and C-H bonds.
C) Alkanes are acyclic or cyclic.
D) Acyclic alkanes have two fewer H atoms than cyclic alkanes with the same number of carbons.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
10
What is the approximate C-C-C bond angle in propane?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds has only primary, secondary and tertiary carbon atoms?
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,2,3-Trimethylpentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds has only primary and secondary carbon atoms?
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,3,3-Trimethylpentane
A) Pentane
B) 2-Methylpentane
C) 2,2-Dimethylpentane
D) 2,3,3-Trimethylpentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
13
What is the hybridization of a carbon atom in an alkane?
A) sp3
B) sp2
C) sp
D) p
A) sp3
B) sp2
C) sp
D) p

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
14
What is the molecular formula of an alkane that has twenty-three carbon atoms?
A) C23H46
B) C23H48
C) C23H50
D) C23H44
A) C23H46
B) C23H48
C) C23H50
D) C23H44

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not another representation for 2-methylbutane? 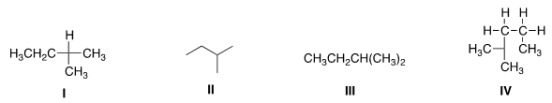
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
16
What is the name of the alkyl group that contains two carbons in a straight chain and one-carbon branch?
A) Ethyl
B) Propyl
C) Isopropyl
D) None of the above
A) Ethyl
B) Propyl
C) Isopropyl
D) None of the above

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
17
How many cycloalkane constitutional isomers (excluding stereoisomers) are there with molecular formula C5H10?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds has only primary and secondary hydrogen atoms?
A) 2-Methylpentane
B) 2,2,3-Trimethylpentane
C) 3-Methylpentane
D) 2,2-Dimethylpentane
A) 2-Methylpentane
B) 2,2,3-Trimethylpentane
C) 3-Methylpentane
D) 2,2-Dimethylpentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements about alkanes is true?
A) Alkanes are aliphatic hydrocarbons having only C-C and C-H bonds.
B) Cyclic alkanes have two fewer H atoms than acyclic alkanes with the same number of carbons.
C) Acyclic alkanes contain carbons joined in one or more rings.
D) Acyclic alkanes have general molecular formula CnH2n.
A) Alkanes are aliphatic hydrocarbons having only C-C and C-H bonds.
B) Cyclic alkanes have two fewer H atoms than acyclic alkanes with the same number of carbons.
C) Acyclic alkanes contain carbons joined in one or more rings.
D) Acyclic alkanes have general molecular formula CnH2n.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
20
What is the molecular formula of a cycloalkane that has six carbon atoms?
A) C6H14
B) C6H10
C) C6H12
D) C6H16
A) C6H14
B) C6H10
C) C6H12
D) C6H16

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name for the following compound? 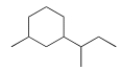
A) 1-Butyl-3-methylcyclohexane
B) 1-sec-Butyl-3-methylcyclohexane
C) 1-Methyl-3-sec-butylcyclohexane
D) 1-sec-Butyl-3-methylhexane

A) 1-Butyl-3-methylcyclohexane
B) 1-sec-Butyl-3-methylcyclohexane
C) 1-Methyl-3-sec-butylcyclohexane
D) 1-sec-Butyl-3-methylhexane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name for the following compound? 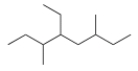
A) 2,3,5-Trimethylhexane
B) 2,4,5-Triethylhexane
C) 2,4-Diethyl-5-methylheptane
D) 4-Ethyl-3,6-dimethyloctane

A) 2,3,5-Trimethylhexane
B) 2,4,5-Triethylhexane
C) 2,4-Diethyl-5-methylheptane
D) 4-Ethyl-3,6-dimethyloctane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
23
What is the IUPAC name for the following compound? 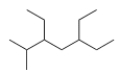
A) 3,5-Diethyl-6-methylheptane
B) 3,5-Diethyl-2-methylheptane
C) 3-Ethyl-5-isopropylheptane
D) 5-Ethyl-3-isopropylheptane

A) 3,5-Diethyl-6-methylheptane
B) 3,5-Diethyl-2-methylheptane
C) 3-Ethyl-5-isopropylheptane
D) 5-Ethyl-3-isopropylheptane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
24
What is the IUPAC name for the following compound? 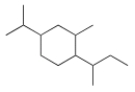
A) 1-sec-Butyl-4-isopropyl-2-methylcyclohexane
B) 1-Isopropyl-3-methyl-4-sec-butylcyclohexane
C) 4-Isopropyl-2-methyl-1-sec-butylcyclohexane
D) 1-sec-Butyl-3-isopropyl-2-methylcyclohexane

A) 1-sec-Butyl-4-isopropyl-2-methylcyclohexane
B) 1-Isopropyl-3-methyl-4-sec-butylcyclohexane
C) 4-Isopropyl-2-methyl-1-sec-butylcyclohexane
D) 1-sec-Butyl-3-isopropyl-2-methylcyclohexane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
25
What is the IUPAC name for the following compound? 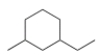
A) 3-Ethyl-1-methylcyclohexane
B) 1-Ethyl-3-methylcyclohexane
C) 1-Ethyl-3-methylhexane
D) 3-Ethyl-1-methylhexane

A) 3-Ethyl-1-methylcyclohexane
B) 1-Ethyl-3-methylcyclohexane
C) 1-Ethyl-3-methylhexane
D) 3-Ethyl-1-methylhexane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following are gauche conformers? 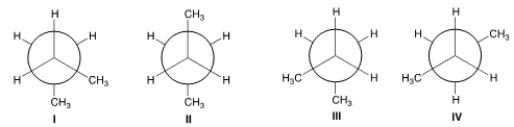
A) I and II
B) I and III
C) II and IV
D) II and III

A) I and II
B) I and III
C) II and IV
D) II and III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name for the following compound? 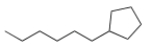
A) Hexylcyclopentane
B) 1-Hexylcyclopentane
C) 1-Cyclopentylhexane
D) 1-Cyclopentylheptane

A) Hexylcyclopentane
B) 1-Hexylcyclopentane
C) 1-Cyclopentylhexane
D) 1-Cyclopentylheptane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements about the conformations of acyclic alkanes is true?
A) Conformations are the same arrangements of atoms that cannot be interconverted by rotation about single bonds.
B) In the eclipsed conformation, the C-H bonds on one carbon bisect the H-C-H bond angle on the adjacent carbon.
C) In the staggered conformation, the C-H bonds on one carbon are directly aligned with the C-H bonds on the adjacent carbon.
D) Rotating the atoms on one carbon by 60° converts an eclipsed conformation into a staggered conformation, and vice versa.
A) Conformations are the same arrangements of atoms that cannot be interconverted by rotation about single bonds.
B) In the eclipsed conformation, the C-H bonds on one carbon bisect the H-C-H bond angle on the adjacent carbon.
C) In the staggered conformation, the C-H bonds on one carbon are directly aligned with the C-H bonds on the adjacent carbon.
D) Rotating the atoms on one carbon by 60° converts an eclipsed conformation into a staggered conformation, and vice versa.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name for the following compound? 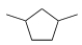
A) 1,4-Dimethylcyclohexane
B) 1,3-Dimethylcyclohexane
C) 1,3-Dimethylcyclopentane
D) 1,4-Dimethylcyclopentane

A) 1,4-Dimethylcyclohexane
B) 1,3-Dimethylcyclohexane
C) 1,3-Dimethylcyclopentane
D) 1,4-Dimethylcyclopentane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
30
Rank the following alkanes in order of decreasing boiling point, putting the alkane with the highest boiling point first. 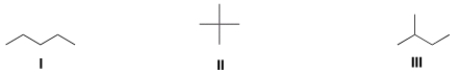
A) I > II > III
B) I > III > II
C) II > III > I
D) III > II > I

A) I > II > III
B) I > III > II
C) II > III > I
D) III > II > I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
31
What is the IUPAC name for the following compound? 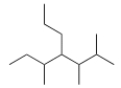
A) 2,3-Dimethyl-4-sec-butylheptane
B) 4-sec-Butyl-2,3-dimethylheptane
C) 3,5,6-Trimethyl-4-propylheptane
D) 2,3,5-Trimethyl-4-propylheptane

A) 2,3-Dimethyl-4-sec-butylheptane
B) 4-sec-Butyl-2,3-dimethylheptane
C) 3,5,6-Trimethyl-4-propylheptane
D) 2,3,5-Trimethyl-4-propylheptane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements about the conformations of acyclic alkanes is not true?
A) The staggered and eclipsed conformations are equally stable.
B) The staggered conformations are more stable than the eclipsed conformations.
C) An energy minimum and maximum occur every 60° as the conformation changes from staggered to eclipsed.
D) Conformations that are neither staggered nor eclipsed are intermediate in energy.
A) The staggered and eclipsed conformations are equally stable.
B) The staggered conformations are more stable than the eclipsed conformations.
C) An energy minimum and maximum occur every 60° as the conformation changes from staggered to eclipsed.
D) Conformations that are neither staggered nor eclipsed are intermediate in energy.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
33
What is the IUPAC name for the following compound? 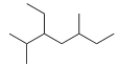
A) 5-Ethyl-3,6-dimethylheptane
B) 3-Ethyl-2,5-dimethylheptane
C) 3-Ethyl-2,5-dimethyloctane
D) 5-Ethyl-3,6-dimethyloctane

A) 5-Ethyl-3,6-dimethylheptane
B) 3-Ethyl-2,5-dimethylheptane
C) 3-Ethyl-2,5-dimethyloctane
D) 5-Ethyl-3,6-dimethyloctane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name for the following compound? 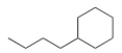
A) 1-Butylcyclohexane
B) Cyclohexanebutane
C) 1-Cyclohexylbutane
D) Butylcyclohexane

A) 1-Butylcyclohexane
B) Cyclohexanebutane
C) 1-Cyclohexylbutane
D) Butylcyclohexane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not a conformer of butane? 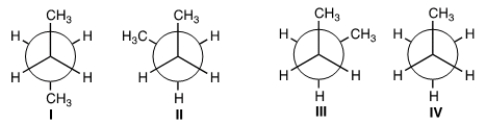
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
36
What is the common name of the following alkyl group? 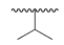
A) Isopropyl
B) Isobutyl
C) sec-Butyl
D) tert-Butyl

A) Isopropyl
B) Isobutyl
C) sec-Butyl
D) tert-Butyl

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name for the following compound? 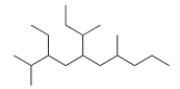
A) 3-Ethyl-2,7-dimethyl-5-sec-butyldecane
B) 5-sec-Butyl-3-ethyl-2,7-dimethyldecane
C) 2,7-Dimethyl-3-ethyl-5-sec-butyldecane
D) 3-Ethyl-2,7-dimethyl-5-isobutyldecane

A) 3-Ethyl-2,7-dimethyl-5-sec-butyldecane
B) 5-sec-Butyl-3-ethyl-2,7-dimethyldecane
C) 2,7-Dimethyl-3-ethyl-5-sec-butyldecane
D) 3-Ethyl-2,7-dimethyl-5-isobutyldecane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
38
Rank the following alkanes in order of increasing melting point, putting the alkane with the lowest melting point first. 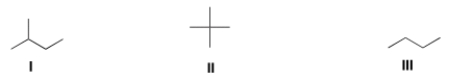
A) I < III < II
B) I < II < III
C) II < III < I
D) III < II < I

A) I < III < II
B) I < II < III
C) II < III < I
D) III < II < I

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following statements about the conformations of acyclic alkanes is true?
A) A staggered conformation with two larger groups 180° from each other is called gauche.
B) Staggered conformations are at energy maxima and eclipsed conformations are energy minima.
C) A staggered conformation with two larger groups 60° from each other is called anti.
D) Gauche conformations are generally higher in energy than anti conformations.
A) A staggered conformation with two larger groups 180° from each other is called gauche.
B) Staggered conformations are at energy maxima and eclipsed conformations are energy minima.
C) A staggered conformation with two larger groups 60° from each other is called anti.
D) Gauche conformations are generally higher in energy than anti conformations.

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
40
What is the common name of the following alkyl group? 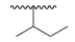
A) Isobutyl
B) sec-Butyl
C) Isopropyl
D) tert-Butyl

A) Isobutyl
B) sec-Butyl
C) Isopropyl
D) tert-Butyl

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is the most stable conformation of trans-1-isopropyl-3-methylcyclohexane? 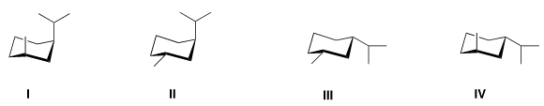
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the highest energy conformer of 2,3-dimethylbutane? 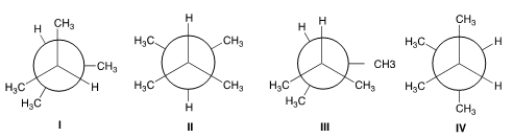
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following chair conformations represents trans-1,3-dimethylcyclohexane? 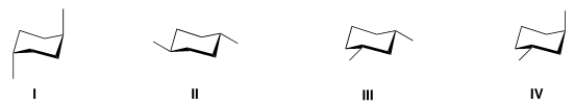
A) I
B) II
C) III
D) IV
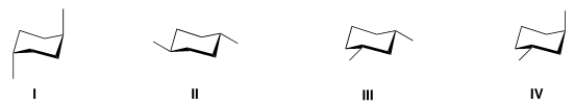
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the most stable conformation of the following compound? 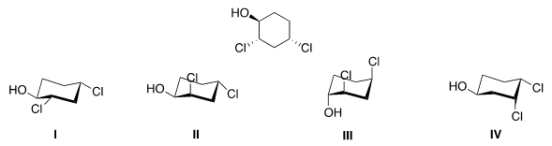
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following cycloalkanes has the most angle strain?
A) Cyclopropane
B) Cyclobutane
C) Cyclopentane
D) Cyclohexane
A) Cyclopropane
B) Cyclobutane
C) Cyclopentane
D) Cyclohexane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following conformers has the highest energy? 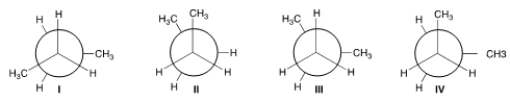
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the most stable conformation of cis-1-ethyl-3-isopropylcyclohexane? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
48
What are the products of the combustion of alkanes?
A) Carbon and hydrogen
B) Carbon and water
C) Carbon dioxide and hydrogen
D) Carbon dioxide and water
A) Carbon and hydrogen
B) Carbon and water
C) Carbon dioxide and hydrogen
D) Carbon dioxide and water

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following cycloalkanes has the least angle strain?
A) Cyclopropane
B) Cyclohexane
C) Cyclopentane
D) Cycloheptane
A) Cyclopropane
B) Cyclohexane
C) Cyclopentane
D) Cycloheptane

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
50
Rank the conformers of 1,2,4-trimethylcyclohexane in order of decreasing stability, putting the most stable first. 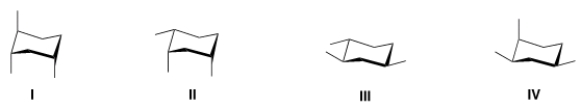
A) I > II > III > IV
B) III > II > IV > I
C) III > IV > II > I
D) I > II > IV > III

A) I > II > III > IV
B) III > II > IV > I
C) III > IV > II > I
D) I > II > IV > III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following chair conformations represents trans-1,4-dimethylcyclohexane? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following are anti conformers? 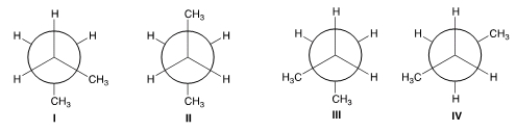
A) I and II
B) II and III
C) I and IV
D) II and IV

A) I and II
B) II and III
C) I and IV
D) II and IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
53
Select the most stable conformer of cis-1,3-cyclohexanediol. 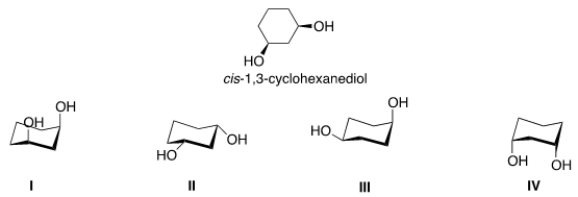
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
54
Rank the conformers of butane in order of decreasing stability, putting the most stable first. 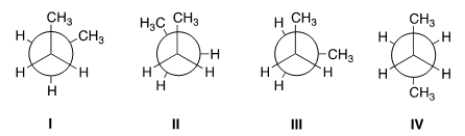
A) IV > I > II > III
B) IV > I > III > II
C) II > III > I > IV
D) I > IV > II > III

A) IV > I > II > III
B) IV > I > III > II
C) II > III > I > IV
D) I > IV > II > III

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the lowest energy conformer of 2,3-dimethybutane? 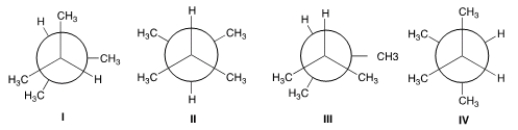
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck
56
What is the alternate chair conformation of the following compound? 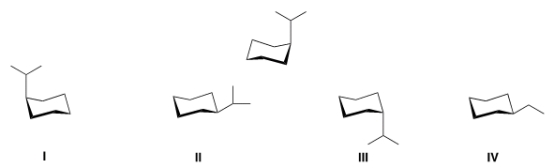
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 56 flashcards in this deck.
Unlock Deck
k this deck


