Deck 18: Electrophilic Aromatic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/51
Play
Full screen (f)
Deck 18: Electrophilic Aromatic Substitution
1
What is a major problem with Friedel-Crafts alkylation?
A) It requires high temperatures.
B) The conditions are too acidic.
C) The starting material is frequently over-alkylated.
D) The products coordinate with the aluminum chloride.
A) It requires high temperatures.
B) The conditions are too acidic.
C) The starting material is frequently over-alkylated.
D) The products coordinate with the aluminum chloride.
The starting material is frequently over-alkylated.
2
What is the electrophile in aromatic sulfonation?
A) H2SO3
B) H2SO4
C) SO3+
D) HSO3+
A) H2SO3
B) H2SO4
C) SO3+
D) HSO3+
HSO3+
3
What is the major organic product obtained from the following reaction? 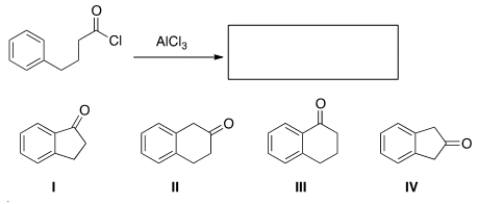
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
III
4
What is the major organic product obtained from the following reaction? 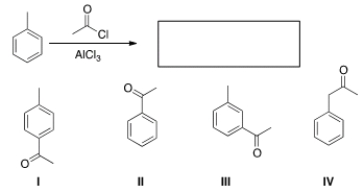
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
5
Why is sulfuric acid used in aromatic nitration?
A) To keep the reaction from getting too basic
B) To form the active electrophile NO2+
C) To protonate the aromatic ring
D) To keep the reaction from getting too acidic
A) To keep the reaction from getting too basic
B) To form the active electrophile NO2+
C) To protonate the aromatic ring
D) To keep the reaction from getting too acidic

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
6
What is the electrophile in the Friedel-Crafts alkylation reaction with tert-butylchloride?
A) the tert-butyl cation
B) a complex of tert-butylchloride and aluminum chloride
C) a proton
D) aluminum chloride
A) the tert-butyl cation
B) a complex of tert-butylchloride and aluminum chloride
C) a proton
D) aluminum chloride

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
7
What is the major organic product obtained from the following reaction? 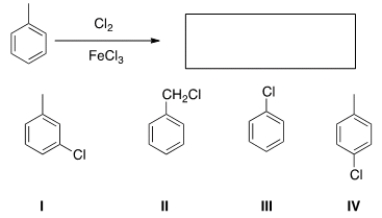
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
8
What is the major organic product obtained from the following reaction? 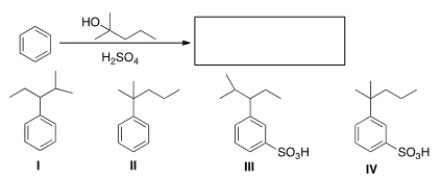
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major organic product obtained from the following reaction? 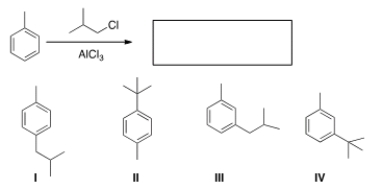
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
10
Why is the nitro group a meta director?
A) Because it is sterically very large.
B) Because it adds electron density to the meta position, thus activating it.
C) Because it stabilizes the intermediate cation.
D) Because it removes more electron density from the ortho and para positions than the meta position, thus deactivating the meta position less.
A) Because it is sterically very large.
B) Because it adds electron density to the meta position, thus activating it.
C) Because it stabilizes the intermediate cation.
D) Because it removes more electron density from the ortho and para positions than the meta position, thus deactivating the meta position less.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about the mechanism of electrophilic aromatic substitution is not true?
A) All electrophilic aromatic substitution reactions occur via a two-step mechanism.
B) The transition state of the first step is lower in energy.
C) The first step is the rate-determining step.
D) The second step is the fast step.
A) All electrophilic aromatic substitution reactions occur via a two-step mechanism.
B) The transition state of the first step is lower in energy.
C) The first step is the rate-determining step.
D) The second step is the fast step.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
12
What are the two effects that have to be considered to determine the influence a substituent will have on electrophilic aromatic substitution?
A) Steric and electronic
B) Inductive and steric
C) Inductive and resonance
D) Resonance and electronic
A) Steric and electronic
B) Inductive and steric
C) Inductive and resonance
D) Resonance and electronic

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
13
What is the major organic product obtained from the following reaction? 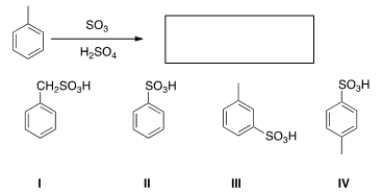
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
14
What is the driving force for losing a proton as the last step in electrophilic aromatic substitution?
A) To neutralize the base that is present
B) To make room for the electrophile
C) To make the ring more reactive
D) To rearomatize the ring system
A) To neutralize the base that is present
B) To make room for the electrophile
C) To make the ring more reactive
D) To rearomatize the ring system

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
15
What will be the site that leads to the major mono substitution product for an electrophilic aromatic substitution reaction of the following compound? 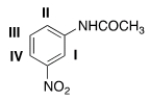
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
16
What is the major organic product obtained from the following reaction? 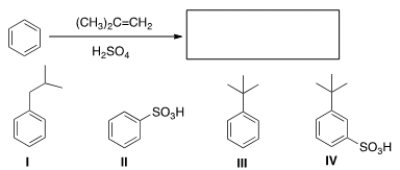
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
17
What is the electrophile in aromatic nitration?
A) NO+
B) NO2+
C) NO3+
D) NO2H
A) NO+
B) NO2+
C) NO3+
D) NO2H

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
18
What is the first step in the general mechanism for electrophilic aromatic substitution?
A) Protonation of the aromatic ring
B) Deprotonation of the aromatic ring
C) Addition of the electrophile to the aromatic ring
D) Loss of the electrophile from the aromatic ring
A) Protonation of the aromatic ring
B) Deprotonation of the aromatic ring
C) Addition of the electrophile to the aromatic ring
D) Loss of the electrophile from the aromatic ring

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following halides will not work as an electrophile in a Friedel-Crafts alkylation reaction? 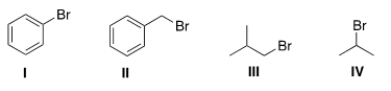
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
20
What is the major organic product obtained from the following reaction? 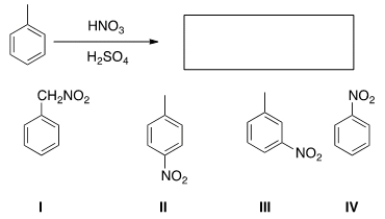
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following substituents are activators in electrophilic aromatic substitution?
A) CH3O
B) Cl
C) NO2
D) HSO3
A) CH3O
B) Cl
C) NO2
D) HSO3

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
22
Rank the following deactivating groups in order of increasing deactivating strength, listing the least deactivating first.
A) I < II < III < IV
B) II < III < IV < I
C) II < I < III < IV
D) IV < III < I < II
A) I < II < III < IV
B) II < III < IV < I
C) II < I < III < IV
D) IV < III < I < II

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
23
Rank the following activating groups in order of decreasing strength of activation, listing the most activating first.
A) IV > II > III > I
B) II > III > IV > I
C) III > IV > II > I
D) II > IV > III > I
A) IV > II > III > I
B) II > III > IV > I
C) III > IV > II > I
D) II > IV > III > I

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
24
What are the product(s) of the following reaction? 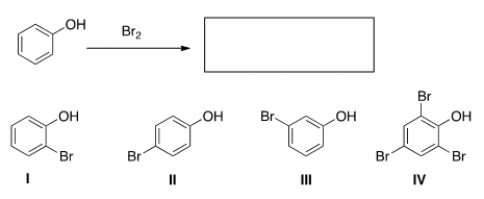
A) Only I and II
B) Only I and III
C) Only II and III
D) Only IV

A) Only I and II
B) Only I and III
C) Only II and III
D) Only IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following substituents is an ortho, para director?
A) " CHO"
B) " COOH"
C) " NHCOR"
D) " CN"
A) " CHO"
B) " COOH"
C) " NHCOR"
D) " CN"

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following substituents is a meta director?
A) " N(CH3)2"
B) " OCH3"
C) " NHCOCH3"
D) " SO3H"
A) " N(CH3)2"
B) " OCH3"
C) " NHCOCH3"
D) " SO3H"

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
27
What is (are) the product(s) of the following reaction? 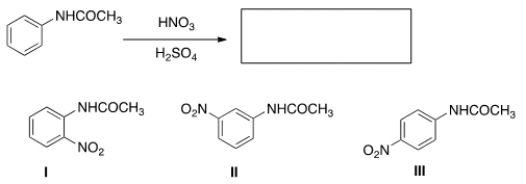
A) Only I
B) Only II
C) Only III
D) Only I and III

A) Only I
B) Only II
C) Only III
D) Only I and III

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
28
What are the product(s) of the following reaction? 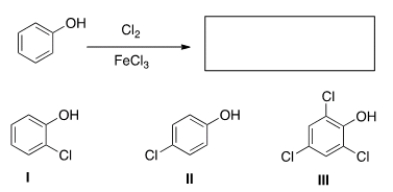
A) Only I
B) Only II
C) Only I and II
D) Only III

A) Only I
B) Only II
C) Only I and II
D) Only III

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
29
Rank the following compounds in order of decreasing reactivity in electrophilic aromatic substitution. 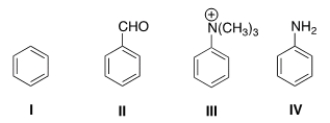
A) II > IV > I > III
B) II > III > IV > I
C) IV > II > I > III
D) IV > I > II > III

A) II > IV > I > III
B) II > III > IV > I
C) IV > II > I > III
D) IV > I > II > III

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements about nucleophilic aromatic substitution is not true?
A) Increasing the electronegativity of the halogen increases the reactivity of the aryl halide.
B) Increasing the number of electron-withdrawing groups increases the reactivity of the aryl halide.
C) Electron-withdrawing groups stabilize the intermediate carbanion, and lower the energy of the transition state.
D) When a nitro group is located meta to the halogen, the negative charge of the intermediate carbanion can be delocalized onto the NO2 group, thus stabilizing it.
A) Increasing the electronegativity of the halogen increases the reactivity of the aryl halide.
B) Increasing the number of electron-withdrawing groups increases the reactivity of the aryl halide.
C) Electron-withdrawing groups stabilize the intermediate carbanion, and lower the energy of the transition state.
D) When a nitro group is located meta to the halogen, the negative charge of the intermediate carbanion can be delocalized onto the NO2 group, thus stabilizing it.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
31
What is the reactive intermediate formed in the elimination-addition mechanism of nucleophilic aromatic substitution?
A) Carbocation
B) Radical
C) Benzyne
D) Carbanion
A) Carbocation
B) Radical
C) Benzyne
D) Carbanion

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
32
What is (are) the product(s) of the following reaction? 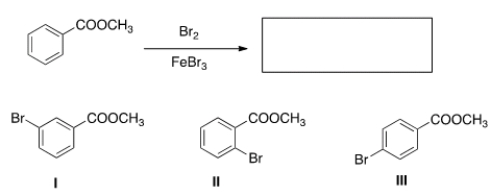
A) Only I
B) Only II
C) Only III
D) Only I and II

A) Only I
B) Only II
C) Only III
D) Only I and II

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
33
How can polyalkylation be minimized in Friedel-Crafts alkylation?
A) Use a large excess of alkyl halide relative to the aromatic compound.
B) Use a large excess of benzene relative to the alkyl halide.
C) Use an alkyl halide without a Lewis acid catalyst.
D) Use a large excess of the Lewis acid catalyst.
A) Use a large excess of alkyl halide relative to the aromatic compound.
B) Use a large excess of benzene relative to the alkyl halide.
C) Use an alkyl halide without a Lewis acid catalyst.
D) Use a large excess of the Lewis acid catalyst.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements about nucleophilic aromatic substitution is true?
A) For the addition-elimination pathway, the nucleophile may become attached either at the site bearing the leaving group or at the site bearing the ortho hydrogen atom.
B) For the elimination-addition pathway, the nucleophile becomes attached only at the site bearing the leaving group.
C) The elimination-addition mechanism is not as common as the addition-elimination mechanism.
D) In the addition-elimination mechanism, the aromatic ring first accepts a pair of electrons from a nucleophile to form a cationic intermediate.
A) For the addition-elimination pathway, the nucleophile may become attached either at the site bearing the leaving group or at the site bearing the ortho hydrogen atom.
B) For the elimination-addition pathway, the nucleophile becomes attached only at the site bearing the leaving group.
C) The elimination-addition mechanism is not as common as the addition-elimination mechanism.
D) In the addition-elimination mechanism, the aromatic ring first accepts a pair of electrons from a nucleophile to form a cationic intermediate.

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
35
Rank the following compounds in order of increasing reactivity in electrophilic aromatic substitution. 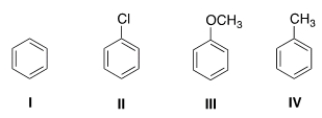
A) I < II < III < IV
B) II < I < IV < III
C) III < IV < I < II
D) II < I < III < IV

A) I < II < III < IV
B) II < I < IV < III
C) III < IV < I < II
D) II < I < III < IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
36
What are the two distinct pathways for nucleophilic aromatic substitution?
A) Addition-substitution and substitution-addition
B) Addition-elimination and elimination-addition
C) Addition-addition and elimination-elimination
D) Elimination-substitution and substitution-elimination
A) Addition-substitution and substitution-addition
B) Addition-elimination and elimination-addition
C) Addition-addition and elimination-elimination
D) Elimination-substitution and substitution-elimination

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following substituents are deactivators in electrophilic aromatic substitution?
A) HO
B) CH3NH
C) CH3O
D) (CH3)3N+
A) HO
B) CH3NH
C) CH3O
D) (CH3)3N+

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
38
What is the major product of the following reaction? 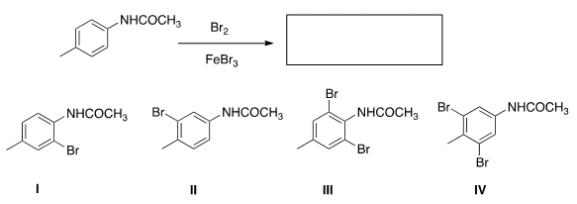
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
39
What is (are) the product(s) of the following reaction? 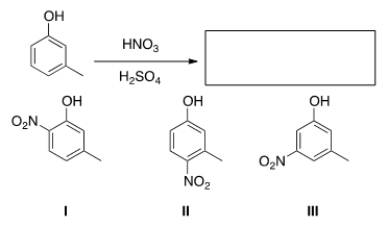
A) Only I
B) Only II
C) Only III
D) Only I and II

A) Only I
B) Only II
C) Only III
D) Only I and II

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
40
What is the reactive intermediate formed in the addition-elimination mechanism of nucleophilic aromatic substitution?
A) Carbocation
B) Radical
C) Benzyne
D) Carbanion
A) Carbocation
B) Radical
C) Benzyne
D) Carbanion

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
41
What is the product of the following sequence of reactions? 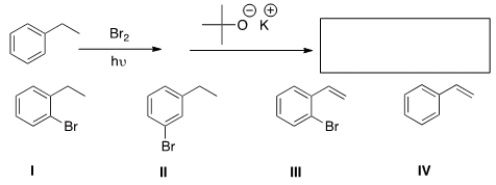
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
42
Which set of reagents would most likely bring about the following transformation? 
A) Br2 and FeBr3
B) NBS and light
C) Br2 in CCl4
D) NaBr and H2O

A) Br2 and FeBr3
B) NBS and light
C) Br2 in CCl4
D) NaBr and H2O

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
43
What is the best choice of reagent to bring about the following transformation? ![<strong>What is the best choice of reagent to bring about the following transformation? </strong> A) [1] LiAlH<sub>4</sub>; [2]H<sub>2</sub>O B) Zn (Hg), HCl C) NH<sub>3</sub>, NaOH D) H<sub>2</sub>, Pd-C](https://storage.examlex.com/TB5871/11ea9088_70b6_ede7_aec7_7998a6e4e744_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] LiAlH4; [2]H2O
B) Zn (Hg), HCl
C) NH3, NaOH
D) H2, Pd-C
![<strong>What is the best choice of reagent to bring about the following transformation? </strong> A) [1] LiAlH<sub>4</sub>; [2]H<sub>2</sub>O B) Zn (Hg), HCl C) NH<sub>3</sub>, NaOH D) H<sub>2</sub>, Pd-C](https://storage.examlex.com/TB5871/11ea9088_70b6_ede7_aec7_7998a6e4e744_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] LiAlH4; [2]H2O
B) Zn (Hg), HCl
C) NH3, NaOH
D) H2, Pd-C

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
44
What is the product of the following reaction? 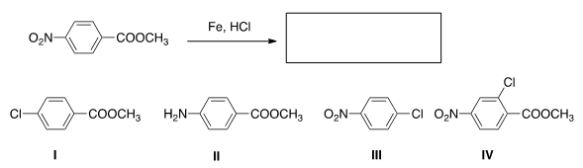
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
45
In addition to the product shown, what other product is formed in the following reaction? 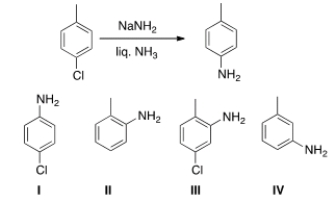
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
46
What is the major product of the following reaction? 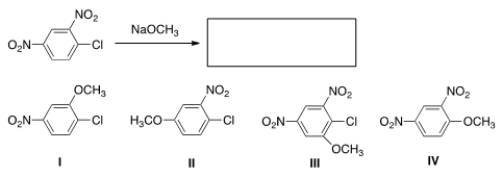
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
47
What is the product of the following reaction? 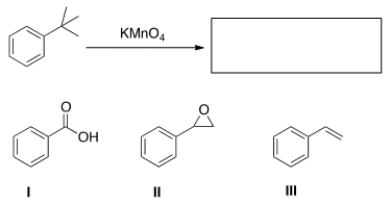
A) I
B) II
C) III
D) None of the above

A) I
B) II
C) III
D) None of the above

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
48
Rank the following compounds in order of increasing reactivity in nucleophilic aromatic substitution. 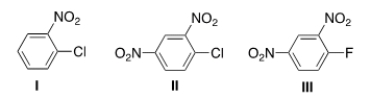
A) III < II < I
B) I < II < III
C) III < I < II
D) I < III < II

A) III < II < I
B) I < II < III
C) III < I < II
D) I < III < II

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
49
What is the product of the following reaction? 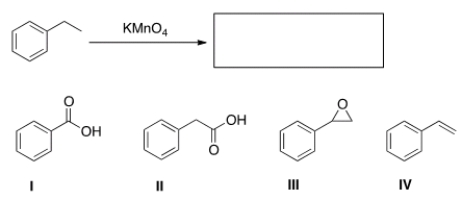
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
50
What is the best choice of reagent to bring about the following transformation? ![<strong>What is the best choice of reagent to bring about the following transformation? </strong> A) [1] LiAlH<sub>4</sub>; [2] H<sub>2</sub>O B) Zn (Hg), HCl C) NH<sub>3</sub>, NaOH D) H<sub>2</sub>, Pd-C](https://storage.examlex.com/TB5871/11ea9088_70b6_51a6_aec7_ebdd27919c12_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] LiAlH4; [2] H2O
B) Zn (Hg), HCl
C) NH3, NaOH
D) H2, Pd-C
![<strong>What is the best choice of reagent to bring about the following transformation? </strong> A) [1] LiAlH<sub>4</sub>; [2] H<sub>2</sub>O B) Zn (Hg), HCl C) NH<sub>3</sub>, NaOH D) H<sub>2</sub>, Pd-C](https://storage.examlex.com/TB5871/11ea9088_70b6_51a6_aec7_ebdd27919c12_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] LiAlH4; [2] H2O
B) Zn (Hg), HCl
C) NH3, NaOH
D) H2, Pd-C

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck
51
Which aryl fluoride reacts the fastest with NaOH? 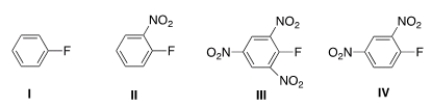
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 51 flashcards in this deck.
Unlock Deck
k this deck


