Deck 1: Structure and Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 1: Structure and Bonding
1
Which of the following statements about bonding is true?
A) Covalent bonds result from the transfer of electrons from one element to another.
B) Ionic bonds result from the transfer of electrons from a metal to a non-metal.
C) Ionic bonds result from the sharing of electrons between two non-metals.
D) Covalent bonds result from the sharing of electrons between two metals.
A) Covalent bonds result from the transfer of electrons from one element to another.
B) Ionic bonds result from the transfer of electrons from a metal to a non-metal.
C) Ionic bonds result from the sharing of electrons between two non-metals.
D) Covalent bonds result from the sharing of electrons between two metals.
Ionic bonds result from the transfer of electrons from a metal to a non-metal.
2
In which of the following ions does carbon have a formal charge?
I II III
A) I
B) II
C) III
D) None of the above
I II III
A) I
B) II
C) III
D) None of the above
None of the above
3
Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules?
A) Carbon forms 4 covalent bonds, nitrogen forms 2 covalent bonds and oxygen forms 3 covalent bonds.
B) Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds and oxygen forms 2 covalent bonds.
C) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds and oxygen forms 2 covalent bonds.
D) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds and oxygen forms 4 covalent bonds.
A) Carbon forms 4 covalent bonds, nitrogen forms 2 covalent bonds and oxygen forms 3 covalent bonds.
B) Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds and oxygen forms 2 covalent bonds.
C) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds and oxygen forms 2 covalent bonds.
D) Carbon forms 4 covalent bonds, nitrogen forms 5 covalent bonds and oxygen forms 4 covalent bonds.
Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds and oxygen forms 2 covalent bonds.
4
Which is the correct Lewis structure for acetic acid (CH3CO2H)? 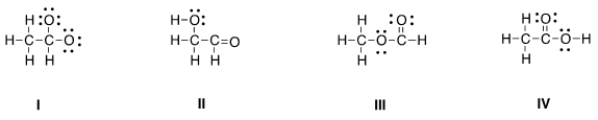
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following Lewis structures is correct? 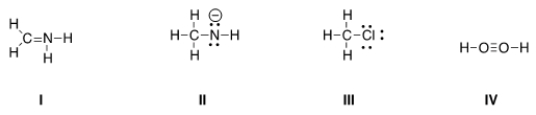
A) I, II
B) I, III
C) II, III
D) III, IV
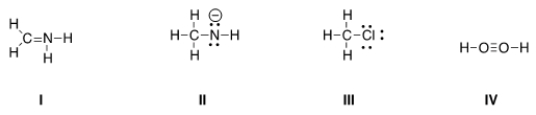
A) I, II
B) I, III
C) II, III
D) III, IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
What is the ground-state electronic configuration of a carbon atom?
A) 1s2, 2s2, 2p5
B) 1s2, 2s2, 2p2
C) 1s2, 2s2, 2p6
D) 1s2, 2s2, 2p4
A) 1s2, 2s2, 2p5
B) 1s2, 2s2, 2p2
C) 1s2, 2s2, 2p6
D) 1s2, 2s2, 2p4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements about constitutional isomers is true?
A) Constitutional isomers are different molecules having different molecular formula.
B) Constitutional isomers are different molecules having same molecular formula.
C) Constitutional isomers are same molecules having different molecular formula.
D) Constitutional isomers are same molecules having the same molecular formula.
A) Constitutional isomers are different molecules having different molecular formula.
B) Constitutional isomers are different molecules having same molecular formula.
C) Constitutional isomers are same molecules having different molecular formula.
D) Constitutional isomers are same molecules having the same molecular formula.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following would you expect to have ionic bonds?
A) CO
B) FBr
C) NF3
D) NaCl
A) CO
B) FBr
C) NF3
D) NaCl

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following Lewis structures is correct? 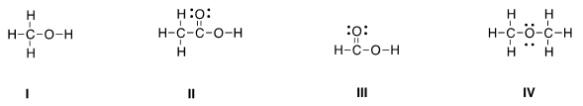
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
What is the ground-state electronic configuration of a fluorine atom?
A) 1s2, 2s2, 2p2
B) 1s2, 2s2, 2p3
C) 1s2, 2s2, 2p4
D) 1s2, 2s2, 2p5
A) 1s2, 2s2, 2p2
B) 1s2, 2s2, 2p3
C) 1s2, 2s2, 2p4
D) 1s2, 2s2, 2p5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
In which of the following ions does carbon have a formal charge?
I II III
A) I
B) II
C) III
D) None of the above
I II III
A) I
B) II
C) III
D) None of the above

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
Which is not an acceptable Lewis structure for the anion CH2NCO-? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following molecules has nonpolar covalent bonds?
A) HCl
B) N2
C) CHCl3
D) NO
A) HCl
B) N2
C) CHCl3
D) NO

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about valence electrons is true?
A) They are the most tightly held electrons.
B) They do not participate in chemical reactions.
C) They are the outermost electrons.
D) They reveal the period number of a second-row element.
A) They are the most tightly held electrons.
B) They do not participate in chemical reactions.
C) They are the outermost electrons.
D) They reveal the period number of a second-row element.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
How many constitutional isomers are there for a molecule having the molecular formula C2H6O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Arrange the following bonds in decreasing order of ionic character, putting the most ionic first.
A) I > II > III > IV
B) IV > II > I > III
C) IV > III > II > I
D) IV > II > III > I
A) I > II > III > IV
B) IV > II > I > III
C) IV > III > II > I
D) IV > II > III > I

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
What is the formal charge of carbon in carbon monoxide (CO) when drawn with a triple bond?
A) 0
B) -2
C) -1
D) +1
A) 0
B) -2
C) -1
D) +1

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules contain both covalent and ionic bonds?
A) I, II
B) I, IV
C) II, III
D) II, IV
A) I, II
B) I, IV
C) II, III
D) II, IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
What is the ground-state electronic configuration of a magnesium cation (Mg2+)?
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s1
C) 1s2, 2s2, 2p6, 3s2
D) 1s2, 2s2, 2p6, 3s2, 3p2
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s1
C) 1s2, 2s2, 2p6, 3s2
D) 1s2, 2s2, 2p6, 3s2, 3p2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
What is the ground-state electronic configuration of a chlorine anion (Cl-)?
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s2, 3p6
C) 1s2, 2s2, 2p6, 3s2, 3p5
D) 1s2, 2s2, 2p6, 3s2, 3p4
A) 1s2, 2s2, 2p6
B) 1s2, 2s2, 2p6, 3s2, 3p6
C) 1s2, 2s2, 2p6, 3s2, 3p5
D) 1s2, 2s2, 2p6, 3s2, 3p4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a resonance structure of the compound below? 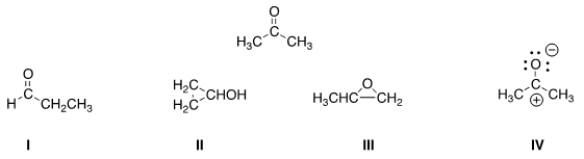
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
How many constitutional isomers are there for a molecule having the molecular C3H8O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
How many different isomers are there for a compound having the molecular formula C3H6O?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
Determine the electron geometry around the indicated atom in each species. 
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
What is the approximate H-C-O bond angle in formaldehyde, H2CO?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
What is the approximate bond angle for the C-C-N bond in acetonitrile, CH3CN?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
Follow the curved arrows to draw the second resonance structure for the ion below. 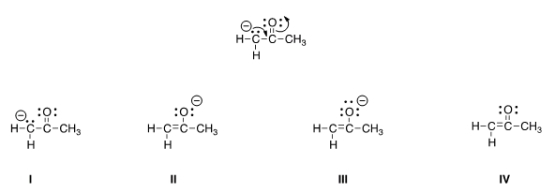
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
How many constitutional isomers are there for a molecule having the molecular formula C2H4Cl2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
What is the approximate C-C-C bond angle in propene, CH3CH=CH2?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Which is more important in each pair of contributing resonance structures? 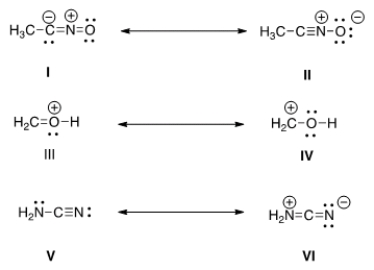
A) II, IV, V
B) II, III, V
C) II, III, VI
D) I, IV, V

A) II, IV, V
B) II, III, V
C) II, III, VI
D) I, IV, V

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following molecules are constitutional isomers?
A) I, II, IV
B) II, III, IV
C) I, III, IV
D) I, II, III
A) I, II, IV
B) II, III, IV
C) I, III, IV
D) I, II, III

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
Rank the following in order of decreasing importance as contributing structures to the resonance hybrid of formaldehyde, H2CO. 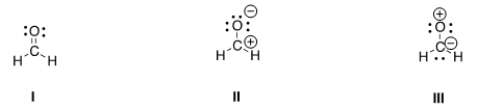
A) I > II > III
B) I > III > II
C) II > I > III
D) III > II > I

A) I > II > III
B) I > III > II
C) II > I > III
D) III > II > I

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following resonance structures is the least important contributor to the resonance hybrid of the formate anion, HCOO-? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following pair does not represent resonance structures? 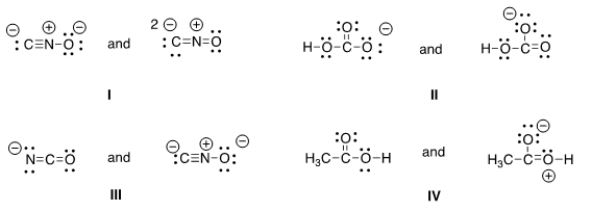
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
What 2 things will change between two resonance structures?
A) The position of multiple bonds and non-bonded electrons.
B) The position of multiple bonds and single bonds.
C) The placement of atoms and single bonds.
D) The placement of atoms and non-bonded electrons.
A) The position of multiple bonds and non-bonded electrons.
B) The position of multiple bonds and single bonds.
C) The placement of atoms and single bonds.
D) The placement of atoms and non-bonded electrons.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
How many constitutional isomers are there for a molecule having the molecular formula C3H6?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements about resonance structures is not true?
A) There is no movement of electrons from one form to another.
B) Resonance structures are not isomers.
C) Resonance structures differ only in the arrangement of electrons.
D) Resonance structures are in equilibrium with each other.
A) There is no movement of electrons from one form to another.
B) Resonance structures are not isomers.
C) Resonance structures differ only in the arrangement of electrons.
D) Resonance structures are in equilibrium with each other.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following compounds has an atom with an unfilled valence shell of electrons?
A) H2O
B) BCl3
C) CH4
D) CO2
A) H2O
B) BCl3
C) CH4
D) CO2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
What is the approximate value of the H-C-H bond angle in methane, CH4?
A) 90°
B) 109.5°
C) 120°
D) 180°
A) 90°
B) 109.5°
C) 120°
D) 180°

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements about resonance structures is true?
A) Resonance structures have the same placement of electrons but different arrangement of atoms.
B) Resonance structures have the same placement of atoms but different arrangement of electrons.
C) Resonance structures have the same placement of atoms and the same arrangement of electrons.
D) Resonance structures have different placement of atoms and different arrangement of electrons.
A) Resonance structures have the same placement of electrons but different arrangement of atoms.
B) Resonance structures have the same placement of atoms but different arrangement of electrons.
C) Resonance structures have the same placement of atoms and the same arrangement of electrons.
D) Resonance structures have different placement of atoms and different arrangement of electrons.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Convert the following skeletal structure to a condensed structure. 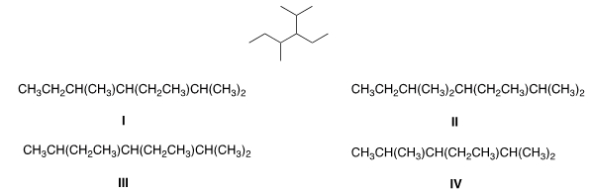
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Avobenzone is an active ingredient in some common sunscreens. Which of the following is the correct molecular formula for avobenzone? 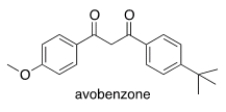
A) C22O22O3
B) C20H22O3
C) C21H23O3
D) C20H24O3

A) C22O22O3
B) C20H22O3
C) C21H23O3
D) C20H24O3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
What is the hybridization for each of the indicated atoms in the following compound? 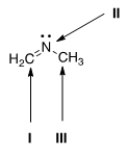
A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.

A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Which atomic orbitals overlap to form the C-H bonding molecular orbitals of ethane, CH3CH3?
A) Csp2 + H1s
B) Csp3 + H1s
C) C2p + H1s
D) Csp + H1s
A) Csp2 + H1s
B) Csp3 + H1s
C) C2p + H1s
D) Csp + H1s

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
In which structure is the hybridization incorrect? 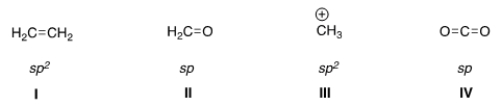
A) I
B) II
C) III
D) IV
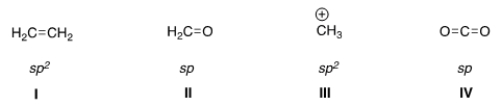
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the appropriate conversion of (CH3)4C to a skeletal structure? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the appropriate conversion of the condensed structure, CH3COCH3, to a Lewis structure? 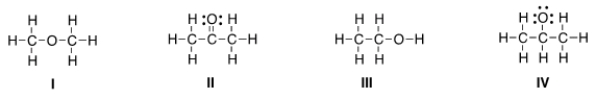
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the appropriate conversion of (CH3)2CHCH2CHClCH3 to a skeletal structure? 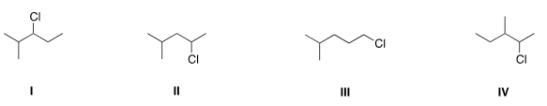
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
What is the order of decreasing bond length for a C-C bond comprised of the following molecular orbitals?
A) I > III > II
B) I > II > III
C) III > II > I
D) II > III > I
A) I > III > II
B) I > II > III
C) III > II > I
D) II > III > I

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements about electronegativity and the periodic table is true?
A) Electronegativity decreases across a row of the periodic table.
B) Electronegativity increases down a column of the periodic table.
C) Electronegativity increases across a row of the periodic table.
D) Electronegativity does not change down a column of the periodic table.
A) Electronegativity decreases across a row of the periodic table.
B) Electronegativity increases down a column of the periodic table.
C) Electronegativity increases across a row of the periodic table.
D) Electronegativity does not change down a column of the periodic table.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Which atomic orbitals overlap to form the carbon-carbon and bonding molecular orbitals of ethylene, H2C=CH2?
A) Csp3 + Csp3, and C2p + C2p
B) Csp3 + Csp3, and Csp2 + Csp2
C) Csp2 + Csp2, and C2p + C2p
D) Csp2 + Csp2, and Csp2 + Csp2
A) Csp3 + Csp3, and C2p + C2p
B) Csp3 + Csp3, and Csp2 + Csp2
C) Csp2 + Csp2, and C2p + C2p
D) Csp2 + Csp2, and Csp2 + Csp2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
Which atomic orbitals overlap to form the carbon-carbon bonding molecular orbital of acetylene, C2H2?
A) Csp2 + Csp2
B) Csp + Csp
C) Csp3 + Csp3
D) C2p + C2p
A) Csp2 + Csp2
B) Csp + Csp
C) Csp3 + Csp3
D) C2p + C2p

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
What is the hybridization of the nitrogen atom in the ammonium cation, NH4+?
A) sp3
B) sp2
C) sp
D) p
A) sp3
B) sp2
C) sp
D) p

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
What is the condensed formula of the compound below? 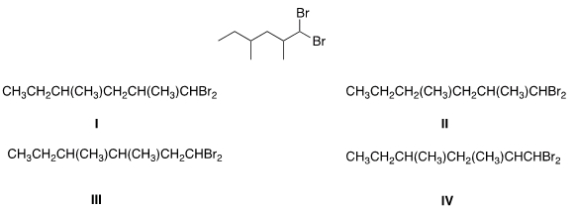
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Rank the following atoms in order of increasing electronegativity, putting the least electronegative first.
A) I < II < III < IV
B) I < IV < II < III
C) III < II < IV < I
D) I < II < IV < III
A) I < II < III < IV
B) I < IV < II < III
C) III < II < IV < I
D) I < II < IV < III

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
What is the hybridization of the carbon atom in the methyl cation, (CH3+)?
A) sp3
B) sp2
C) sp
D) p
A) sp3
B) sp2
C) sp
D) p

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Which atomic orbitals overlap to form the C-H bonding molecular orbitals of ethylene, H2C=CH2?
A) C2p + H1s
B) Csp + H1s
C) Csp3 + H1s
D) Csp2 + H1s
A) C2p + H1s
B) Csp + H1s
C) Csp3 + H1s
D) Csp2 + H1s

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is the appropriate conversion of (CH3)2CHOCH2CH2CH2OH to a skeletal structure? 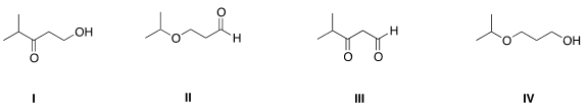
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
When forming molecular orbitals from atomic orbitals, what is the order of increasing C-H bond strength for the following. 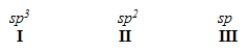
A) II < I < III
B) III < I < II
C) III < II < I
D) I < II < III
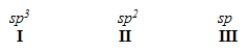
A) II < I < III
B) III < I < II
C) III < II < I
D) I < II < III

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Which atomic orbitals overlap to form the C-H bonding molecular orbitals of acetylene, C2H2?
A) Csp + H1s
B) C2p +H1s
C) Csp3 + H1s
D) Csp2 + H1s
A) Csp + H1s
B) C2p +H1s
C) Csp3 + H1s
D) Csp2 + H1s

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Which molecule has the greatest difference in electronegativity (E) between the two different elements?
A) CO2
B) H2S
C) NH3
D) H2O
A) CO2
B) H2S
C) NH3
D) H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following molecules has non-polar covalent bonds?
A) CO2
B) N2
C) CCl4
D) HF
A) CO2
B) N2
C) CCl4
D) HF

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following molecules has the smallest dipole moment?
A) CO2
B) HCl
C) H2O
D) NH3
A) CO2
B) HCl
C) H2O
D) NH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following compounds are non-polar?
A) I, IV
B) I, II
C) II, III
D) II, IV
A) I, IV
B) I, II
C) II, III
D) II, IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following covalent bonds has the largest dipole moment?
A) C-H
B) C-C
C) C-O
D) H-F
A) C-H
B) C-C
C) C-O
D) H-F

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following molecules does not have a net dipole moment of zero?
A) CCl4
B) BF3
C) CO2
D) NH3
A) CCl4
B) BF3
C) CO2
D) NH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following molecules has a net dipole moment of zero? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Which compound contains the most polar bond?
I II III IV
A) I
B) II
C) III
D) IV
I II III IV
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following molecules has polar covalent bonds?
A) MgO
B) NH3
C) Cl2
D) NaBr
A) MgO
B) NH3
C) Cl2
D) NaBr

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Rank the following atoms in order of decreasing electronegativity, putting the most electronegative first.
A) I > IV > II > III
B) II > III > IV > I
C) III > IV > II > I
D) III > II > IV > I
A) I > IV > II > III
B) II > III > IV > I
C) III > IV > II > I
D) III > II > IV > I

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


