Deck 11: Using Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/36
Play
Full screen (f)
Deck 11: Using Energy
1
What is the maximum efficiency of an engine operating between a reservoir in which ice and water coexist,and a reservoir in which water and steam coexist? The pressure is constant at 1.0 atmosphere for both.
A)27%
B)0.27%
C)100%
D)1.0%
A)27%
B)0.27%
C)100%
D)1.0%
27%
2
A heat pump with a COP of 4.90000007 absorbs heat from the atmosphere at a rate of 30 kW.At what rate is it doing work?
A)6 kW
B)147 kW
C)117 kW
D)36 kW
A)6 kW
B)147 kW
C)117 kW
D)36 kW
6 kW
3
A heat engine has a maximum possible efficiency of 0.800.If it operates between a deep lake with a constant temperature of 287.0 K and a hot reservoir,what is the temperature of the hot reservoir?
A)1440 K
B)359 K
C)517 K
D)646 K
A)1440 K
B)359 K
C)517 K
D)646 K
1440 K
4
A nuclear power plant has an actual efficiency of 39%.If 0.24999999 MW of energy are released from fission,how much electric power does the power plant produce?
A)0.097 MW
B)9.7 MW
C)35 MW
D)0.35 MW
A)0.097 MW
B)9.7 MW
C)35 MW
D)0.35 MW

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
5
What is the average kinetic energy of an ideal gas at 842 K? (The value of Boltzmann's constant is 1.38 × 10-23 J/K. )
A)1.74 × 10-20 J
B)5.81 × 10-21 J
C)1.18 × 10-17 J
D)3.93 × 10-19 J
A)1.74 × 10-20 J
B)5.81 × 10-21 J
C)1.18 × 10-17 J
D)3.93 × 10-19 J

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
6
A person reading a European newspaper finds that the low temperature the night before in Stockholm was -16°C.What is the equivalent temperature in degrees Fahrenheit?
A)3.20000076°F
B)-28.799999°F
C)23.1°F
D)8.9°F
A)3.20000076°F
B)-28.799999°F
C)23.1°F
D)8.9°F

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
7
A system has a heat source supplying heat at a rate of 187 W and is doing work at a rate of 130.899998 W.At what rate is the internal energy of the system changing?
A)56.1000022 W
B)317.900009 W
C)-56.100002 W
D)187 W
A)56.1000022 W
B)317.900009 W
C)-56.100002 W
D)187 W

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
8
A Carnot engine extracts 508 J of heat from a high-temperature reservoir during each cycle,and rejects 303 J of heat to a low-temperature reservoir during the same cycle.What is the efficiency of the engine?
A)0.4
B)0.68
C)1.68
D)0.63
A)0.4
B)0.68
C)1.68
D)0.63

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
9
A heat engine that is 40% efficient and has a heat input of 200 J per cycle at the hot reservoir will waste 80 J of heat each cycle.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
10
A perfectly insulated system has work done by it at a rate of 13 W.At what rate is the internal energy of the system changing?
A)- 13 W
B)13 W
C)0 W
D)6.5 W
A)- 13 W
B)13 W
C)0 W
D)6.5 W

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
11
A Carnot engine operating between a warmer unknown temperature and a reservoir of boiling helium at 1.76 K has an efficiency of 11.0%.What is the warmer temperature?
A)1.98 K
B)0.180 K
C)157 K
D)0.160 K
A)1.98 K
B)0.180 K
C)157 K
D)0.160 K

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
12
A Carnot-efficiency engine operating between a reservoir of liquid mercury at its melting point and a colder reservoir extracts 15.0 J of heat from the mercury and does 4.0 J of work during each cycle.What is the temperature of the colder reservoir? Mercury melts at 233 K.
A)171 K
B)62 K
C)47 K
D)67 K
A)171 K
B)62 K
C)47 K
D)67 K

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
13
A laboratory experiment is to be run at 42.0 K.Express this temperature in Celsius to the nearest degree.
A)-231°C
B)315°C
C)74°C
D)415°C
A)-231°C
B)315°C
C)74°C
D)415°C

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
14
A refrigerator with a COP of 3.7 cools to 9.0°C.What is the high temperature,Th,needed to operate the refrigerator at 9.0°C?
A)85°C
B)1052°C
C)11°C
D)42°C
A)85°C
B)1052°C
C)11°C
D)42°C

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
15
A thermometer flashes the temperature in Celsius and Fahrenheit along with the time.If it flashes 25°F,what will the thermometer flash in Celsius?
A)-3.9°C
B)-18.1°C
C)77°C
D)13.9°C
A)-3.9°C
B)-18.1°C
C)77°C
D)13.9°C

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
16
A heat engine operates between Tc = 294 K and Th = 450 K.What is the maximum possible efficiency?
A)0.35
B)0.65
C)0.53
D)1.53
A)0.35
B)0.65
C)0.53
D)1.53

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
17
A Carnot engine operates between a high temperature reservoir at 435 K and a river with water at 280 K.If it absorbs 3700 J of heat each cycle,how much work per cycle does it perform?
A)1318 J
B)2382 J
C)1449 J
D)2251 J
A)1318 J
B)2382 J
C)1449 J
D)2251 J

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
18
You have a flask of liquid nitrogen at a temperature of -243°C.You then heat the nitrogen until its temperature is doubled.What is the new temperature?
A)486°C
B)-213°C
C)-486°C
D)None of the above
A)486°C
B)-213°C
C)-486°C
D)None of the above

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
19
A fluid in an insulated,flexible bottle is heated by a high resistance wire and expands.If 9 kJ of heat is applied to the system and it does 5 kJ of work,how much does the internal energy change?
A)4 kJ
B)14 kJ
C)-4 kJ
D)45 kJ
A)4 kJ
B)14 kJ
C)-4 kJ
D)45 kJ

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
20
A Carnot engine operates between two reservoirs with unknown temperatures.If the Carnot engine operates at 43% efficiency,what is the ratio of the kelvin temperatures of the reservoirs,Tc/Th?
A)0.57
B)0.0021
C)0.0016
D)0.42999999
A)0.57
B)0.0021
C)0.0016
D)0.42999999

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
21
Just as a heat engine is more and more effective as its efficiency approaches 1,a refrigerator is more and more effective as its coefficient of performance approaches 1.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
22
Water turns to ice when it is placed in a freezer.During this process,the entropy of this water has decreased.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
23
Is liberating heat through combustion a reversible or an irreversible process?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
24
An important feature of the Carnot cycle is that
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used,and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.
A)its efficiency can be 100%.
B)its efficiency depends only on the absolute temperature of the hot reservoir used.
C)its efficiency is determined by the temperatures of the hot and cold reservoirs between which it works and by the properties of the working substance used,and on nothing else.
D)it is an example of an irreversible process that can be analyzed exactly without approximations.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
25
All real heat engines are less efficient than the Carnot engine.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
26
A refrigerator increases the entropy of its hot reservoir at the same time as it decreases the entropy of its cold reservoir,but a heat engine does just the opposite.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
27
A 20% efficient heat engine that must do 50 J of work each cycle requires a heat input of 250 J per cycle.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following relationships are true for all types of heat engines? (There may be more than one correct choice. )
A)e = 1 -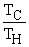
B)e =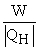
C)e =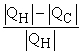
D)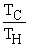 =
=
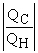
E)e = 1 -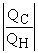
A)e = 1 -
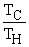
B)e =
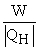
C)e =
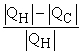
D)
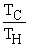 =
=
E)e = 1 -


Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a TRUE statement?
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one,depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into heat.
D)It is impossible to transfer heat from a cooler to a hotter body.
E)All of these statements are false.
A)The second law of thermodynamics is a consequence of the first law of thermodynamics.
B)It is possible for heat to flow spontaneously from a hot body to a cold one or from a cold one to a hot one,depending on whether or not the process is reversible or irreversible.
C)It is not possible to convert work entirely into heat.
D)It is impossible to transfer heat from a cooler to a hotter body.
E)All of these statements are false.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
30
A Carnot engine operating between heat reservoirs at 10°C and 40°C has an efficiency of 75%.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is a FALSE statement?
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system is conserved,i.e. ,constant.
D)Entropy can be measured in units of J/K.
A)Entropy is a quantitative measure of disorder.
B)The total entropy change in one cycle of a Carnot engine is zero.
C)The entropy of an isolated system is conserved,i.e. ,constant.
D)Entropy can be measured in units of J/K.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
32
If two different gases are at the same temperature,the average translational kinetic energy per molecule must be the same for both of them.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
33
As you increase the difference between the temperatures of the hot and cold reservoirs,a Carnot engine converts a larger and larger percent of the heat put into it into work.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
34
Heat can be transformed into work with 100% efficiency,but work cannot be transformed into heat with 100% efficiency.

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
35
Does an increase in entropy increase or decrease the ability of a system to do work?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck
36
Is temperature a macroscopic or a microscopic concept?

Unlock Deck
Unlock for access to all 36 flashcards in this deck.
Unlock Deck
k this deck


