Deck 12: Thermal Properties of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 12: Thermal Properties of Matter
1
A 960.0 g iron meteor impacts the earth at a speed of 1268 m/s.If its kinetic energy is entirely converted to heat of the meteorite,what will the resultant temperature rise be? (The specific heat for iron is 113 cal/kg · °C. )
A)1700°C
B)1,630,000°C
C)1.80°C
D)7110°C
A)1700°C
B)1,630,000°C
C)1.80°C
D)7110°C
1700°C
2
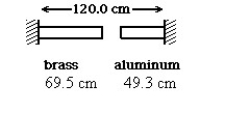 A brass rod is 69.5 cm long and an aluminum rod is 49.3 cm long when both rods are at an initial temperature of 0°C.The rods are placed in line with a gap of 1.2 cm between them.The distance between the far ends of the rods is maintained at 120.0 cm throughout.The temperature is raised until the two rods are barely in contact.The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 and 2.4 × 10-5 K-1 ,respectively.In the figure,the temperature at which contact of the rods barely occurs,in °C,is closest to:
A brass rod is 69.5 cm long and an aluminum rod is 49.3 cm long when both rods are at an initial temperature of 0°C.The rods are placed in line with a gap of 1.2 cm between them.The distance between the far ends of the rods is maintained at 120.0 cm throughout.The temperature is raised until the two rods are barely in contact.The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 and 2.4 × 10-5 K-1 ,respectively.In the figure,the temperature at which contact of the rods barely occurs,in °C,is closest to:A)466
B)443
C)420
D)490
E)513
466
3
A gas follows the pV trajectory shown in the figure.How much work is done per cycle by the gas if p0 = 5.4 atm? 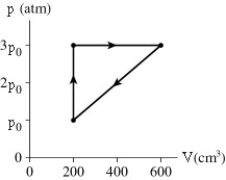
A)220 J
B)440 J
C)870 J
D)1100 J

A)220 J
B)440 J
C)870 J
D)1100 J
220 J
4
An ideal gas is in a closed container.If its pressure is 133 Pa initially,and its temperature is 20.0ºC,what is its pressure after its temperature is raised to 60.0ºC?
A)151 Pa
B)117 Pa
C)44 Pa
D)399 Pa
A)151 Pa
B)117 Pa
C)44 Pa
D)399 Pa

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
A .20 kg ice cube at 0.0°C has sufficient heat added to it to cause total melting,and the resulting water is heated to 70.0ºC.How much heat is added? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg. )
A)130 kJ
B)14,000 kJ
C)81 kJ
D)59 kJ
A)130 kJ
B)14,000 kJ
C)81 kJ
D)59 kJ

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
A cold trap is set up to cause molecules to linger near the suction in a vacuum system.If the cold trap has an effective volume of .200 L and is maintained at 13.0 K,how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 mol-1,and the universal gas constant is 8.314 J/K · mol.Assume the behavior of an ideal gas. )
A)1.11 × 1019 molecules
B)1.10 × 1022 molecules
C)7.71 × 1020 molecules
D)7.71 × 1023 molecules
A)1.11 × 1019 molecules
B)1.10 × 1022 molecules
C)7.71 × 1020 molecules
D)7.71 × 1023 molecules

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
A cylinder fitted with a movable piston contains ideal gas at 27°C,pressure 0.50 x 105 Pa and volume 1.25 m3.What will be the final temperature if the gas is compressed to 0.80 m3 and the pressure rises to 0.82 × 105 Pa?
A)41.8°C
B)67.7°C
C)125°C
D)246°C
E)154°C
A)41.8°C
B)67.7°C
C)125°C
D)246°C
E)154°C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
A sphere of surface area 1.25 m2 and emissivity 1.0 is at a temperature of 100.0°C.The rate at which it radiates heat into empty space is closest to
A)7.1 W
B)0.71 mW
C)1.4 kW
D)9.9 mW
E)3.7 W
A)7.1 W
B)0.71 mW
C)1.4 kW
D)9.9 mW
E)3.7 W

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
A constant-volume gas thermometer supports a 33 mm high column of mercury when its temperature is 216 K.What will the height of the column of mercury be when the temperature is 298 K? Answer to the nearest millimeter.
A)46 mm
B)24 mm
C)36 mm
D)42 mm
A)46 mm
B)24 mm
C)36 mm
D)42 mm

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
How many moles of an ideal gas are there in a container with a pressure of 108,893 Pa,a temperature of 321 K,and a volume of 2.0 L? The universal gas constant is 8.314 J/K · mol.
A)0.082 mol
B)82 mol
C)5600 mol
D)11,000 mol
A)0.082 mol
B)82 mol
C)5600 mol
D)11,000 mol

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
An aluminum rod is 10.0 cm long and a steel rod is 80.0 cm long when both rods are at a temperature of 15°C.Both rods have the same diameter.The rods are joined end-to-end to form a rod 60.0 cm long.The coefficients of linear expansion of aluminum and steel are 2.4 x 10-5 K-1,and 1.2 × 10-5 K-1,respectively.The temperature is raised to 90°C.The increase in the length of the joined rod,in mm,is closest to:
A)0.90
B)0.81
C)0.72
D)0.63
E)0.99
A)0.90
B)0.81
C)0.72
D)0.63
E)0.99

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
A heat conducting rod,1.60 m long,is made of an aluminum section,0.90 m long,and a copper section,0.70 m long.Both sections have a cross-sectional area of 0.0004 m2.The aluminum end and the copper end are maintained at temperatures of 30°C and 170°C,respectively.The thermal conductivities of aluminum and copper are 205 and 385 W/m ∙ K,respectively.The rate at which heat is conducted in the rod is closest to:
A)9.0 W
B)7.9 W
C)10 W
D)11 W
E)12 W
A)9.0 W
B)7.9 W
C)10 W
D)11 W
E)12 W

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
A 24 liter tank contains ideal helium gas at 27°C and a pressure of 22.0 Atm.How many moles of gas are in the tank?
A)238 moles
B)138 moles
C)17.5 moles
D)21.5 moles
E)76.0 moles
A)238 moles
B)138 moles
C)17.5 moles
D)21.5 moles
E)76.0 moles

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
A glass flask has a volume of 500 ml at a temperature of 20°C.The flask contains 492 ml of mercury at an equilibrium temperature of 20°C.The temperature is raised until the mercury reaches the 500 ml reference mark.The coefficients of volume expansion of mercury and glass are 18 × 10-5 K-1 and 2.0 × 10-5 K-1,respectively.The temperature at which this occurs,in °C,is closest to:
A)122
B)112
C)102
D)110
E)132
A)122
B)112
C)102
D)110
E)132

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
A constant-volume gas thermometer is filled with air whose pressure is 104 Pa at the triple point of water.What would the pressure be at 32.0 K?
A)12.2 Pa
B)1.13 × 10-3 Pa
C)888 Pa
D)84.1 Pa
A)12.2 Pa
B)1.13 × 10-3 Pa
C)888 Pa
D)84.1 Pa

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
A constant-volume gas thermometer has a pressure of 132.48 Pa at the triple point of water.What is the change of pressure that occurs for each one degree Kelvin change of temperature?
A)0.48 Pa
B)132.48 Pa
C)131.52 Pa
D)2.07 Pa
A)0.48 Pa
B)132.48 Pa
C)131.52 Pa
D)2.07 Pa

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
A monatomic ideal gas (Cv = 3/2 R)undergoes an isothermal expansion at 300 K,as the volume increased from 0.05 m3 to 0.15 m3.The final pressure is 130 kPa.The heat transfer to the gas,in kJ,is closest to:
A)21
B)11
C)-21
D)-11
E)zero
A)21
B)11
C)-21
D)-11
E)zero

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
A 2294 kg sample of water at 0°C is cooled to -36ºC,and freezes in the process.How much heat is liberated? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg.The specific heat for ice is 2050 J/kg · K. )
A)935,000 kJ
B)597,000 kJ
C)1,110,000 kJ
D)334,000 kJ
A)935,000 kJ
B)597,000 kJ
C)1,110,000 kJ
D)334,000 kJ

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
A sealed 29 m3 tank is filled with 9000 moles of ideal oxygen gas (diatomic)at an initial temperature of 270 K.The gas is heated to a final temperature of 330 K.The atomic mass of oxygen is 16.0 g/mol.The initial pressure of the gas,in MPa,is closest to:
A)0.70
B)0.79
C)0.88
D)0.61
E)0.51
A)0.70
B)0.79
C)0.88
D)0.61
E)0.51

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
A hot air balloon has a volume of 2000 m3 when fully inflated,and the air inside the balloon is always at atmospheric pressure because of the large opening used to fill the balloon and heat the air inside it.What's the mass of hot air inside the balloon if its temperature is 120°C? (Assume a molecular weight of 28.8 g/mole for air. )
A)1790 kg
B)5850 kg
C)203 kg
D)62.0 kg
E)None of the above
A)1790 kg
B)5850 kg
C)203 kg
D)62.0 kg
E)None of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
1.0 mol of an elemental solid and 1.0 mol of a monatomic gas interact thermally.What is the temperature change of the solid if the gas temperature decreases by 60°C at constant volume?
A)30°C increase
B)30°C decrease
C)60°C increase
D)60°C decrease
A)30°C increase
B)30°C decrease
C)60°C increase
D)60°C decrease

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
The figure shows 0.0074 mol of gas that undergoes the process 1 → 2 → 3.What is the volume V3? 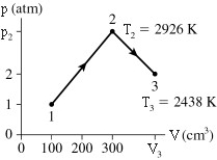
A)740 cm3
B)630 cm3
C)510 cm3
D)400 cm3

A)740 cm3
B)630 cm3
C)510 cm3
D)400 cm3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
A 920 g empty iron kettle is put on a stove.How much heat in joules must it absorb to raise its temperature form 15.0ºC to 93.0ºC? (The specific heat for iron is 113 cal/kg · ºC. )
A)33,900 J
B)40,500 J
C)8110 J
D)40,100 J
A)33,900 J
B)40,500 J
C)8110 J
D)40,100 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
A 24.0 kg sample of ice is at 0.00°C.How much heat is needed to melt it? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg. )
A)8.02 × 103 kJ
B)2.19 × 106 kJ
C)0.00 kJ
D)5.42 × 104 kJ
A)8.02 × 103 kJ
B)2.19 × 106 kJ
C)0.00 kJ
D)5.42 × 104 kJ

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
The diagrams shows a PV diagram for 4.3 g of oxygen gas in a sealed container.The temperature of state 1 is 21°C.What are the temperatures T3 and T4? 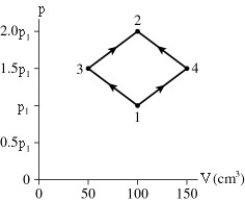
A)-52°C and 390°C
B)16°C and 47°C
C)220°C and 660°C
D)11°C and 32°C

A)-52°C and 390°C
B)16°C and 47°C
C)220°C and 660°C
D)11°C and 32°C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
0.20 g of hydrogen gas are held in a rigid container.The temperature of the gas is changed from 50 K to 80 K.How much heat is needed?
A)37 J
B)25 J
C)50 J
D)75 J
A)37 J
B)25 J
C)50 J
D)75 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
Some properties of glass are listed here.  A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?
A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?
A)3.1 × 107 J
B)3.1 × 104 J
C)8.6 × 103 J
D)8.6 J
E)3.1 × 105 J
 A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?
A glass window pane is 2.7 m high,2.4 m wide and 9 mm thick.The temperature at the inner surface of the glass is 19ºC and at the outer surface 4°C.How much heat is lost each hour through the window?A)3.1 × 107 J
B)3.1 × 104 J
C)8.6 × 103 J
D)8.6 J
E)3.1 × 105 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
148.0 g of water is heated using 67.0 W of power,with perfect efficiency.How long will it take to raise the temperature of the water from 15ºC to 25ºC?
A)92 s
B)5.3 s
C)22 s
D)410,000 s
A)92 s
B)5.3 s
C)22 s
D)410,000 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
A person consumes a large meal containing 14 kcal.What is the power this meal produces if it is to be "burned off" due to exercise in 6 hours?
A)2.7 W
B)9763 W
C)0.6 W
D)0.0027 W
A)2.7 W
B)9763 W
C)0.6 W
D)0.0027 W

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
A 7 kg sample of mercury is completely solidified and liberates 81.06 kJ of energy.What is the original temperature of the mercury? (The melting point of mercury is 234 K,the heat of fusion of mercury is 11.3 kJ/kg,and the specific heat of mercury is 140 J/kg·K. )
A)240 K
B)580 K
C)2.0 K
D)850 K
A)240 K
B)580 K
C)2.0 K
D)850 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
As 771.0 kg copper bar is put into a smelter for melting.The initial temperature of the copper is 300.0 K.How much heat must the smelter produce to completely melt the copper bar? (The specific heat for copper is 386 J/kg · K,the heat of fusion for copper is 205 kJ/kg,and its melting point is 1357 K. )
A)4.73 × 105 kJ
B)3.15 × 1011 kJ
C)3.15 × 108 kJ
D)5.62 × 105 kJ
A)4.73 × 105 kJ
B)3.15 × 1011 kJ
C)3.15 × 108 kJ
D)5.62 × 105 kJ

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
A heat conducting rod,0.90 m long,is made of an aluminum section,0.20 m long,and a copper section,0.70 m long.Both sections have a cross-sectional area of 0.0004 m2.The aluminum end and the copper end are maintained at temperatures of 30ºC and 230ºC,respectively.The thermal conductivities of aluminum and copper are 205 and 385 W/m ∙ K,respectively.The temperature of the aluminum-copper junction in the rod,in ºC,is closest to:
A)100
B)93
C)86
D)80
E)74
A)100
B)93
C)86
D)80
E)74

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
An object having an emissivity 0.725 radiates heat at a rate of 10 W when it is at a temperature T.If its temperature is doubled,it will radiate at a rate of
A)160 W
B)80 W
C)40 W
D)20 W
A)160 W
B)80 W
C)40 W
D)20 W

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
The figure shows a PV diagram for 8.3 g of nitrogen gas in a sealed container.The temperature of state 1 is 79°C.What are (a)pressure p1 and (b)temperature T2? 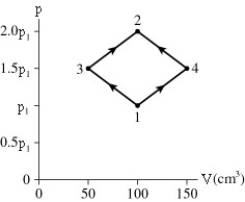
A)(a)86 atm (b)700°C
B)(a)19 atm (b)700°C
C)(a)86 atm (b)160°C
D)(a)19 atm (b)160°C

A)(a)86 atm (b)700°C
B)(a)19 atm (b)700°C
C)(a)86 atm (b)160°C
D)(a)19 atm (b)160°C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
A glass beaker of unknown mass contains 65.0 mL of water.The system absorbs 2000.0 cal of heat and the temperature rises 20.0ºC as a result.What is the mass of the beaker? (The specific heat for glass is .18 cal/g · ºC. )
A)190 g
B)560 g
C)540 g
D)270,000 g
A)190 g
B)560 g
C)540 g
D)270,000 g

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
A container with rigid walls is filled with 4 mol of air with Cv = 2.5R.How much does the internal energy change if the temperature rises from 16ºC to 437ºC?
A)35,000 J
B)421 J
C)3500 J
D)8750 J
A)35,000 J
B)421 J
C)3500 J
D)8750 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
At what temperature would the average thermal speed of oxygen molecules be 13.0 m/s? Oxygen is assumed to approximate an ideal gas.The mass of one O2 molecule is 5.312 × 10-26 kg.
A)0.217 K
B)1800 K
C)5410 K
D)1.31 × 1023 K
A)0.217 K
B)1800 K
C)5410 K
D)1.31 × 1023 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Heat is added to a 2.0 kg piece of ice at a rate of 793.0 kW.How long will it take for the ice at 0.0ºC to melt? (For water Lf = 334 kJ/kg and Lv = 2257 kJ/kg. )
A)0.84 s
B)530,000 s
C)0.0 s
D)670 s
A)0.84 s
B)530,000 s
C)0.0 s
D)670 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
The figure shows a 50 kg lead cylindrical piston that floats on 0.68 mol of compressed air at 30°C.How far does the piston move if the temperature is increased to 300°C? 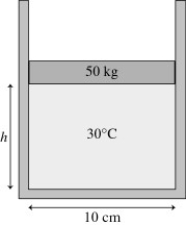
A)120 cm
B)250 cm
C)130 cm
D)1300 cm

A)120 cm
B)250 cm
C)130 cm
D)1300 cm

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
114.0 g of water is heated using 67.0 W of power,with perfect efficiency.How long will it take to raise the temperature of the water from 15ºC to 25ºC?
A)71 s
B)4.1 s
C)17 s
D)320,000 s
A)71 s
B)4.1 s
C)17 s
D)320,000 s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
If you drop an ice cube at 0°C into boiling water,the ice will not change temperature until it has melted.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
52 g of methane (CH4)contain 3.25 moles of this gas.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
A horizontal line on a PV diagram represents an isobaric process.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
What is the average speed of oxygen molecules at 154 K? Oxygen is assumed to approximate an ideal gas.The mass of one O2 molecule is 5.312 × 10-26 kg.
A)346 m/s
B)2.19 m/s
C)8.15 × 1011 m/s
D)6.33 m/s
A)346 m/s
B)2.19 m/s
C)8.15 × 1011 m/s
D)6.33 m/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
An ideal gas goes from point A to point B on a PV diagram.If A and B are at the same temperature,then they must be the same point.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
As you add heat to boiling water,its temperature gets higher and higher.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
The amount of heat needed to raise the temperature of one gram of an ideal gas by 1.0 K at constant volume is the same for all monatomic gases.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
The pressure of an ideal gas in a sealed rigid container is directly proportional to its temperature only if the temperature is measured in K.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
Doubling the root mean square speed of the molecules of a gas has a greater effect on its pressure than doubling the number of gas molecules in the container.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
If two different gases are at the same temperature,the root mean square speed of their molecules must be the same for both of them.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
An oxygen molecule falls in a vacuum.From what height must it fall so that its kinetic energy at the bottom equals the average energy of an oxygen molecule at 800 K?
A)31,800 meters
B)10,600 meters
C)21,100 meters
D)42,300 meters
A)31,800 meters
B)10,600 meters
C)21,100 meters
D)42,300 meters

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
In an isothermal process,the gas temperature always remains constant and the PV diagram is a hyperbola.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
Dust particles are pulverized rock,which has density 2500kg/m3.They are approximately spheres with 10μm in diameter.Treating dust as an ideal gas,what is the rms speed of a dust particle at 400°C?
A)5.2 × 10-5 m/s
B)1.7 × 10-5 m/s
C)3.0 × 10-5 m/s
D)7.3 × 10-5 m/s
A)5.2 × 10-5 m/s
B)1.7 × 10-5 m/s
C)3.0 × 10-5 m/s
D)7.3 × 10-5 m/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
When you compress a gas,the molecules get smaller.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
Two objects,A and B,are not in thermal equilibrium with each other.Object C is in thermal equilibrium with object A.What can you say about objects B and C with respect to thermal equilibrium?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
18 g of water (H2O)contain 6.02 × 1023 atoms of hydrogen.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
If the root mean square speed of gas molecules is doubled,then both its pressure and its absolute temperature will also be doubled.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
The amount of heat needed to raise the temperature of one mole of an ideal gas by 1.0 K at constant volume is the same for all monatomic gases.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
At 0°C molecular motion ceases.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
The temperature is increased from 20°C to 180°C.By what factor does the rms speed of a molecule change?
A)1.2
B)1.5
C)3.0
D)2.4
A)1.2
B)1.5
C)3.0
D)2.4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
Which contains more moles of material: 80 grams of helium or 400 grams of argon?
A)the helium
B)the argon
C)Both contain the same number of moles.
A)the helium
B)the argon
C)Both contain the same number of moles.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
You may have noticed that when you get out of a swimming pool and stand dripping wet in a light breeze,you feel much colder than you feel after you dry off.Why is this?
A)The moisture on your skin has good thermal conductivity.
B)Water has a relatively large heat capacity.
C)This is a purely psychological effect resulting from the way in which sensory nerves in the skin are stimulated.
D)The water on your skin is colder than the surrounding air.
E)540 calories of heat are required to evaporate each gram of water from your skin,and most of this heat flows out of your body.
A)The moisture on your skin has good thermal conductivity.
B)Water has a relatively large heat capacity.
C)This is a purely psychological effect resulting from the way in which sensory nerves in the skin are stimulated.
D)The water on your skin is colder than the surrounding air.
E)540 calories of heat are required to evaporate each gram of water from your skin,and most of this heat flows out of your body.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a FALSE statement?
A)Heat is energy transferred into or out of a system as a result of a temperature difference between the system and its surroundings.
B)The heat added to an ideal gas during the transition from state 1 to state 2 depends only on the initial and final states,1 and 2,and not on the path by which the gas went from one to the other.
C)When a gas goes from one state to another,the work done depends on the path followed.
D)It does not make sense to refer to "the amount of heat in a body."
E)When an ideal gas experiences a free expansion,its temperature doesn't change.
A)Heat is energy transferred into or out of a system as a result of a temperature difference between the system and its surroundings.
B)The heat added to an ideal gas during the transition from state 1 to state 2 depends only on the initial and final states,1 and 2,and not on the path by which the gas went from one to the other.
C)When a gas goes from one state to another,the work done depends on the path followed.
D)It does not make sense to refer to "the amount of heat in a body."
E)When an ideal gas experiences a free expansion,its temperature doesn't change.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
If a piece of metal has a hole in it and the metal is heated,how does the area of the hole change?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is a true statement?
A)Several days after a snowstorm,the roof on Jones's house is uniformly covered with snow,whereas on Smith's house next door the snow has completely melted off.A likely reason for this is that Smith's house has better roof insulation than does Jones's.
B)When you get out of a swimming pool and stand dripping wet in a breeze you feel colder than you would if your skin were dry.This is a purely psychological effect,since measurement of your skin temperature would yield the same value in both cases,wet or dry.
C)In some regions the following sign is frequently seen as one approaches a bridge: "CAUTION: BRIDGE MAY BE ICY." One reason you might expect a bridge to be icier than the road leading up to it is that the bridge has both its top and bottom exposed to cold air,whereas only the top surface of the road is in contact with cold air.
D)When the metal cooling coils in a freezer become coated with ice this helps the freezer more effectively remove heat from warm foods placed in the freezer.
E)Thermos bottles have a vacuum between the inner and outer layers of glass in order to reduce heat transfer due to radiation,since radiation cannot travel through a vacuum.
A)Several days after a snowstorm,the roof on Jones's house is uniformly covered with snow,whereas on Smith's house next door the snow has completely melted off.A likely reason for this is that Smith's house has better roof insulation than does Jones's.
B)When you get out of a swimming pool and stand dripping wet in a breeze you feel colder than you would if your skin were dry.This is a purely psychological effect,since measurement of your skin temperature would yield the same value in both cases,wet or dry.
C)In some regions the following sign is frequently seen as one approaches a bridge: "CAUTION: BRIDGE MAY BE ICY." One reason you might expect a bridge to be icier than the road leading up to it is that the bridge has both its top and bottom exposed to cold air,whereas only the top surface of the road is in contact with cold air.
D)When the metal cooling coils in a freezer become coated with ice this helps the freezer more effectively remove heat from warm foods placed in the freezer.
E)Thermos bottles have a vacuum between the inner and outer layers of glass in order to reduce heat transfer due to radiation,since radiation cannot travel through a vacuum.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
An ideal gas is heated.If the gas is in a container that prevents its volume from changing,what happens to the pressure?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
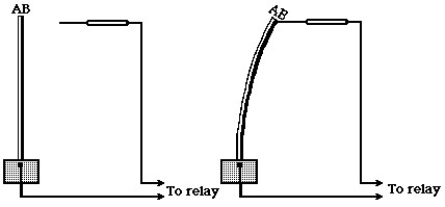
Shown here is a device that can be used to turn a furnace on or off, depending on the temperature sensed by the device.
In the figure,the principle underlying the operation of this device is that
A)different metals have different latent heats.
B)different metals have different thermal conductivities.
C)different metals have different thermal expansion coefficients.
D)heat always flows from hot to cold,never from cold to hot.
E)different metals have different heat capacities.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it,which of the following would be most helpful to know?
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
An air bubble is formed at the bottom of a lake,and begins rising toward the surface.The air is hotter than the water.If the temperature of the air remains constant as the bubble rises,the volume of the bubble will
A)increase.
B)decrease.
C)remain constant.
A)increase.
B)decrease.
C)remain constant.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
An ideal gas inside box A of volume V has a temperature T and contains N molecules.An ideal gas inside box B of volume V/2 has a temperature T/2,and also contains N molecules.In which box is the pressure the highest?
A)Box A
B)Box B
C)The pressure is the same in both boxes.
A)Box A
B)Box B
C)The pressure is the same in both boxes.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
The oxygen molecules and the nitrogen molecules at any one place in our atmosphere are at the same temperature.How do the root-mean-square speeds of the two molecules compare?
A)same
B)N2 > O2
C)O2 > N2
A)same
B)N2 > O2
C)O2 > N2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
For an ideal gas the internal energy U depends only on
A)volume.
B)temperature.
C)pressure.
A)volume.
B)temperature.
C)pressure.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
What measures the average kinetic energy associated with random translational motion of molecules?

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
In the equation pV = nRT,
A)T is measured in degrees Celsius.
B)R has different values for different gases.
C)V is the velocity of the flowing gas.
D)n is the number of moles of gas.
A)T is measured in degrees Celsius.
B)R has different values for different gases.
C)V is the velocity of the flowing gas.
D)n is the number of moles of gas.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
An ideal gas is held in a container of volume V at pressure P.The rms speed of a gas molecule under these conditions is v.If now the volume and pressure are changed to 2V and 2P,the rms speed of a molecule will be:
A)1/2 v
B)v
C)2v
D)4v
E)v/4
A)1/2 v
B)v
C)2v
D)4v
E)v/4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
In years of heavy snow pack in the mountains it is sometimes desirable to induce early melting of the snow,rather than wait until it all melts suddenly and causes floods.It has been suggested that a way to accomplish this might be to have planes fly over the snow fields and sprinkle them with black soot.What do you think of this idea? Would it work?
A)Yes,it would probably work because the black surface would be a better absorber of sunlight than would the white snow.
B)Yes,it would work because the soot would raise the melting point of the snow.
C)Yes,it would work because the soot would decrease the specific heat capacity of the snow.
D)No,it would not work because it is infrared radiation and not visible radiation which melts the snow.
E)No,it would not work because sunlight has very little effect on how fast the snow melts.
A)Yes,it would probably work because the black surface would be a better absorber of sunlight than would the white snow.
B)Yes,it would work because the soot would raise the melting point of the snow.
C)Yes,it would work because the soot would decrease the specific heat capacity of the snow.
D)No,it would not work because it is infrared radiation and not visible radiation which melts the snow.
E)No,it would not work because sunlight has very little effect on how fast the snow melts.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
What is the total translational kinetic energy in a classroom filled with nitrogen at 1.01 × 105 Pa and 20.7ºC? The dimensions of the classroom are 4.60 m × 5.20 m × 8.80 m.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
A nail penetrates into wood when hammered more easily than the hammer (if it misses the nail)because
A)the pressure of the nail on the wood is higher than the pressure of the hammer on the wood.
B)the force of the nail on the wood is higher than the force of the hammer on the wood.
C)Both of the above statements are true.
A)the pressure of the nail on the wood is higher than the pressure of the hammer on the wood.
B)the force of the nail on the wood is higher than the force of the hammer on the wood.
C)Both of the above statements are true.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
A flask contains a mixture of argon and neon gases.The root-mean-square speed of the argon gas is determined to be 1210 m/s.What is the root-mean-square speed of the neon gas? The atomic masses are argon,39.95 g/mole;neon,20.18 g/mole.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


