Deck 29: Atoms and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/48
Play
Full screen (f)
Deck 29: Atoms and Molecules
1
A hydrogen atom is in its n = 2 excited state when its electron absorbs 9.5 eV in an interaction with a photon.What is the energy of the resulting free electron?
A)5.1 eV
B)6.6 eV
C)6.9 eV
D)7.6 eV
A)5.1 eV
B)6.6 eV
C)6.9 eV
D)7.6 eV
5.1 eV
2
Light excites atomic hydrogen from its lowest level to the n = 12 level.What is the energy of the light?
A)13.7 eV
B)32.2 eV
C)61.4 eV
D)91.8 eV
A)13.7 eV
B)32.2 eV
C)61.4 eV
D)91.8 eV
13.7 eV
3
The electron in a hydrogen atom is infinitely distant at zero potential energy and is at an average radius of ao = 0.0529 nm when bound by 13.6 eV.A barely bound H atom may have an average radius as large as a bacterium,R = 14 μm.What is the nearest principal quantum number in this state?
A)514
B)51
C)16
D)264,650
A)514
B)51
C)16
D)264,650
514
4
In the spectrum of hydrogen the lines obtained by setting m = 1 in Balmer's formula is called the Lyman series.Calculate the wavelength of the spectral line of the 16th member of the Lyman series.
A)91.6 nm
B)371 nm
C)102 nm
D)83.2 nm
A)91.6 nm
B)371 nm
C)102 nm
D)83.2 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
5
The shortest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is n = 3,is closest to:
A)820 nm
B)850 nm
C)880 nm
D)910 nm
E)940 nm
A)820 nm
B)850 nm
C)880 nm
D)910 nm
E)940 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
6
The longest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is n = 3,is closest to:
A)550 nm
B)575 nm
C)600 nm
D)625 nm
E)650 nm
A)550 nm
B)575 nm
C)600 nm
D)625 nm
E)650 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
7
The shortest wavelength of a photon that can be emitted by a hydrogen atom,for which the initial state is n = 12,is closest to:
A)92 nm
B)82 nm
C)72 nm
D)62 nm
E)52 nm
A)92 nm
B)82 nm
C)72 nm
D)62 nm
E)52 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
8
An atom has completely filled inner shells and a single valence electron in an excited p state.The filled inner shells have an orbital momentum equal to zero.The magnitude of the orbital angular momentum of the atom is closest to:
A)1.0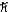
B)1.2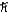
C)1.4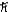
D)1.7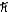
E)2.0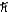
A)1.0
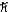
B)1.2
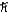
C)1.4
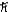
D)1.7
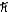
E)2.0

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
9
The longest wavelength of a photon that can be emitted by a hydrogen atom,for which the final state is n = 9,is closest to:
A)39,000 nm
B)22,000 nm
C)7400 nm
D)16,000 nm
E)28,000 nm
A)39,000 nm
B)22,000 nm
C)7400 nm
D)16,000 nm
E)28,000 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
10
A hydrogen atom is excited to the n = 10 level.Its decay to the n = 4 level is detected in a photographic plate.What is the frequency of the light photographed?
A)1.73 × 1014 Hz
B)1740 Hz
C)1740 nm
D)1.46 × 10-9 km
A)1.73 × 1014 Hz
B)1740 Hz
C)1740 nm
D)1.46 × 10-9 km

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
11
Radio astronomers often study the radiation emitted by a hydrogen atom from a transition between the two hyperfine levels associated with the ground state.This radiation has a wavelength of 21 cm.What is the energy difference between the hyperfine levels?
A)5.92 × 10-6 eV
B)5.92 × 10-25 J
C)1.66 × 10-24 J
D)4.73 × 10-25 J
A)5.92 × 10-6 eV
B)5.92 × 10-25 J
C)1.66 × 10-24 J
D)4.73 × 10-25 J

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
12
The energy required to remove the electron from a hydrogen atom in the n = 11 state is closest to:
A)0.11 eV
B)0.094 eV
C)0.080 eV
D)0.17 eV
E)0.14 eV
A)0.11 eV
B)0.094 eV
C)0.080 eV
D)0.17 eV
E)0.14 eV

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
13
One of the emission lines described by the original version of Balmer's formula has wavelength 372 nm.What is the value of n in Balmer's formula that gives this emission line?
A)14
B)15
C)16
D)17
A)14
B)15
C)16
D)17

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
14
Light shines through atomic hydrogen gas.It is seen that the gas absorbs light readily at a wavelength of 91.33 nm.What is the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level.
A)25
B)27
C)22
D)32
A)25
B)27
C)22
D)32

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
15
Find the energy of the photon emitted when an electron drops from the n = 20 state to the n = 7 state in a hydrogen atom.
A)0.244 eV
B)0.263 eV
C)0.283 eV
D)0.302 eV
A)0.244 eV
B)0.263 eV
C)0.283 eV
D)0.302 eV

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the orbital Bohr radius of the n = 3 excited state in a hydrogen atom.
A)0.476 nm
B)0.159 nm
C)0.381 nm
D)0.548 nm
A)0.476 nm
B)0.159 nm
C)0.381 nm
D)0.548 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
17
A hydrogen atom makes a downward transition from the n = 20 state to the n = 5 state.Find the wavelength of the emitted photon.
A)2.43 μm
B)1.46 μm
C)1.94 μm
D)2.92 μm
A)2.43 μm
B)1.46 μm
C)1.94 μm
D)2.92 μm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
18
What is the wavelength of the light emitted by atomic Hydrogen according to Balmer's formula with m = 3 and n = 8?
A)955 nm
B)389 nm
C)1950 nm
D)384 nm
A)955 nm
B)389 nm
C)1950 nm
D)384 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
19
A hydrogen atom is excited to the n = 6 level.Its decay to the n = 2 level is detected in a photographic plate.What is the wavelength of the detected emission?
A)410 nm
B)93.8 nm
C)1090 nm
D)93.1 nm
A)410 nm
B)93.8 nm
C)1090 nm
D)93.1 nm

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
20
What is the frequency of the light emitted by atomic Hydrogen according to Balmer's formula with m = 8 and n = 12?
A)2.86 × 1013 Hz
B)10,500 Hz
C)5830 nm
D)5.83 × 10-6 m
A)2.86 × 1013 Hz
B)10,500 Hz
C)5830 nm
D)5.83 × 10-6 m

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
21
It is impossible for an electron to lose its spin angular momentum.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
22
In atoms having two or more electrons,no two electrons can have the same value for all four quantum numbers.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
23
In atoms having more than one electron,electrons can only make transitions for which the l quantum number changes by one unit.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
24
The electron cloud of an atom is formed because the electron spreads out in space.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
25
All atoms of a given element contain exactly the same number of protons in their nucleus,but they may have different numbers of neutrons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
26
When collisional excitation occurs,an electron collides with an atom and transfers all of its energy to that atom.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
27
14C and 14N both contain 8 neutrons in their nuclei.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
28
When stimulated emission occurs,the emitted photon and the incident (stimulating)photon have exactly the same energy and travel in the same direction.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
29
In spontaneous emission,the electrons all spontaneously emit photons of the same wavelength.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
30
All stationary states of the hydrogen atom for which n = 4 have the same energy.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
31
The number of electrons in the nucleus is equal to the number of protons.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
32
Although the electron has an inherent spin angular momentum,this does not mean that it is actually a spinning ball of matter.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
33
According to the Bohr model of the atom,an electron can only jump from a high to a low energy state if it emits a photon whose energy is the same as the energy difference between those two states.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
34
How many 3d electron states can an atom have?
A)0
B)4
C)6
D)8
E)10
A)0
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
35
The word "laser" is taken from Light Amplification by Stimulated Emission of Radiation.What is meant by "stimulated"?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the statements below is true?
A)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a smaller range of distances from the proton.
B)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a larger range of distances from the proton.
C)For a particular shell,an electron in a hydrogen atom with a higher angular momentum will have a higher mechanical energy.
D)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found farther from the proton.
A)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a smaller range of distances from the proton.
B)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found in a larger range of distances from the proton.
C)For a particular shell,an electron in a hydrogen atom with a higher angular momentum will have a higher mechanical energy.
D)For a particular shell,an electron in a hydrogen atom with a higher angular momentum is more likely to be found farther from the proton.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
37
Do all transitions (excluding fine and hyperfine transitions)that end up in the ground state of hydrogen give ultraviolet light? Why?

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
38
How many 2d electron states can an atom have?
A)0
B)4
C)6
D)8
E)10
A)0
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
39
Neon has 10 electrons.The element of next higher Z that has chemical properties very similar to those of neon has Z equal to:
A)11
B)17
C)18
D)19
E)36
A)11
B)17
C)18
D)19
E)36

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
40
No two distinct atoms produce the same emission spectrum.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
41
The Bohr model of the hydrogen atom was not able to explain
A)the cause of why some emission lines were brighter than other emission lines.
B)the wavelengths of the emission lines in the ultraviolet range.
C)the wavelengths of the emission lines in the infrared range.
D)None of the above;the Bohr model explained all of these features.
A)the cause of why some emission lines were brighter than other emission lines.
B)the wavelengths of the emission lines in the ultraviolet range.
C)the wavelengths of the emission lines in the infrared range.
D)None of the above;the Bohr model explained all of these features.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
42
In Bohr's model of the hydrogen atom,electrons orbiting the proton with a high orbital radius have
A)less kinetic energy,more potential energy,and more mechanical energy than those with a low orbital radius.
B)less kinetic energy,more potential energy,and the same mechanical energy than those with a low orbital radius.
C)more kinetic energy,less potential energy,and more mechanical energy than those with a low orbital radius.
D)less kinetic energy,less potential energy,and less mechanical energy than those with a low orbital radius.
A)less kinetic energy,more potential energy,and more mechanical energy than those with a low orbital radius.
B)less kinetic energy,more potential energy,and the same mechanical energy than those with a low orbital radius.
C)more kinetic energy,less potential energy,and more mechanical energy than those with a low orbital radius.
D)less kinetic energy,less potential energy,and less mechanical energy than those with a low orbital radius.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
43
An electron and a proton are both moving such that their kinetic energy is roughly 100 eV.How do the speeds of the particles compare?
A)The electron is moving about 30 times faster than the proton.
B)The electron is moving about 1000 times faster than the proton.
C)The electron and proton are moving about the same speed.
D)The proton is moving about 30 times faster than the electron.
A)The electron is moving about 30 times faster than the proton.
B)The electron is moving about 1000 times faster than the proton.
C)The electron and proton are moving about the same speed.
D)The proton is moving about 30 times faster than the electron.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
44
How many electrons can be found with principal quantum number n = 3 in a suitably heavy atom?
A)18
B)6
C)20
D)9
A)18
B)6
C)20
D)9

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
45
Suppose Rutherford were able to somehow control the speed of the alpha particles he fired toward the gold nucleus.If he were able to double the speed of the alpha particles,the ones that are scattered backwards would get __________ times closer to the gold nucleus.
A)two
B)three
C)four
A)two
B)three
C)four

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
46
Consider an atom with the electron configuration 1s22s22p63s23p6.Which of the following is an accurate statement concerning this atom?
A)The atomic number of this atom is Z = 11.
B)This atom is in an excited state.
C)This atom has a non-zero angular momentum.
D)This atom is most likely to give rise to an ion with charge +2e.
E)This atom would probably be very inert chemically.
A)The atomic number of this atom is Z = 11.
B)This atom is in an excited state.
C)This atom has a non-zero angular momentum.
D)This atom is most likely to give rise to an ion with charge +2e.
E)This atom would probably be very inert chemically.

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
47
The correct ground state electron configuration of boron (Z = 5)is:
A)1s22s22p
B)1s22s2p3
C)1s21p22s
D)1s22p23s
E)1s22p3
A)1s22s22p
B)1s22s2p3
C)1s21p22s
D)1s22p23s
E)1s22p3

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck
48
What is the greatest magnitude of the orbital angular momentum L that you can find in a state with n = 6?
A)5.48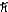
B)5.92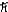
C)6
D)6.48
A)5.48
B)5.92
C)6
D)6.48

Unlock Deck
Unlock for access to all 48 flashcards in this deck.
Unlock Deck
k this deck


