Deck 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/131
Play
Full screen (f)
Deck 19: Synthesis and Reactions of Beta-Dicarbonyl Compounds: More Chemistry of Enolate Ions
1
What is the product of the Dieckmann condensation of this diester, 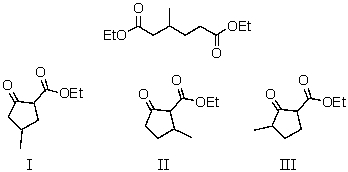
A) I
B) II
C) III
D) I and II
E) I,II,and III

A) I
B) II
C) III
D) I and II
E) I,II,and III
I and II
2
What would be the product of the following sequence? 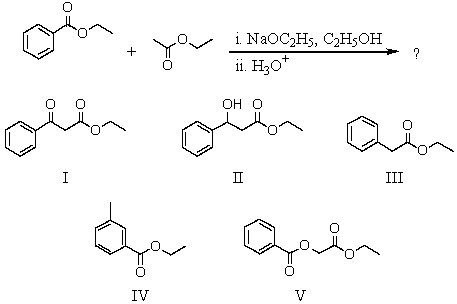
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
3
Why is CH3ONa not used in the Claisen condensation of ethyl acetate?
A) CH3O- is a weaker base than the CH3CH2O- which is used.
B) CH3O-Na+ is more difficult to prepare than CH3CH2O-Na+.
C) CH3O- would abstract a proton from the ethyl group of the ester.
D) Use of CH3O-Na+ would result in transesterification.
E) CH3O-Na+ can be used as well as CH3CH2O-Na+.
A) CH3O- is a weaker base than the CH3CH2O- which is used.
B) CH3O-Na+ is more difficult to prepare than CH3CH2O-Na+.
C) CH3O- would abstract a proton from the ethyl group of the ester.
D) Use of CH3O-Na+ would result in transesterification.
E) CH3O-Na+ can be used as well as CH3CH2O-Na+.
Use of CH3O-Na+ would result in transesterification.
4
What is the product of the Dieckmann condensation of this diester, 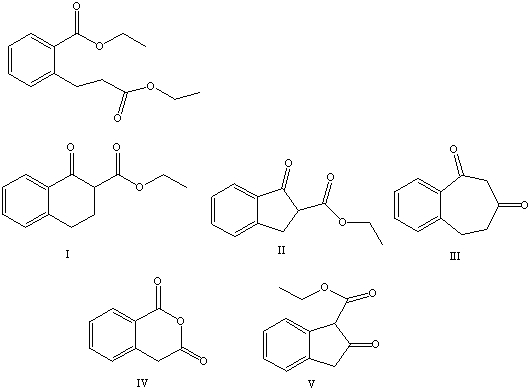
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
5
The product(s)of the reaction of 2 mol of ethyl butanoate with sodium ethoxide is(are):
A)
B)
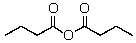
C)
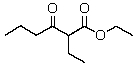
D)
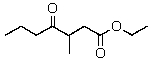
E)
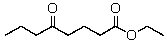
A)
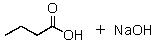
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the synthesis below: What is compound X? 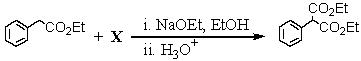
A) O=C(OEt)2
B) HCO2Et
C) EtO-CO-CO-OEt
D) CH3CO2Et
E) BrCH2CO2Et
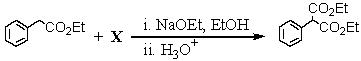
A) O=C(OEt)2
B) HCO2Et
C) EtO-CO-CO-OEt
D) CH3CO2Et
E) BrCH2CO2Et

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
7
What is the product of the Dieckmann condensation of this diester, 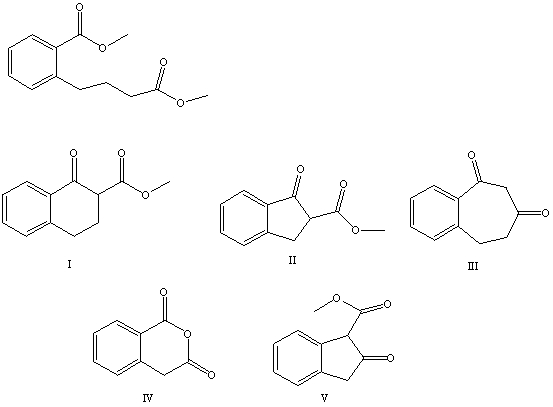
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
8
What is the product of the Dieckmann condensation of this diester, 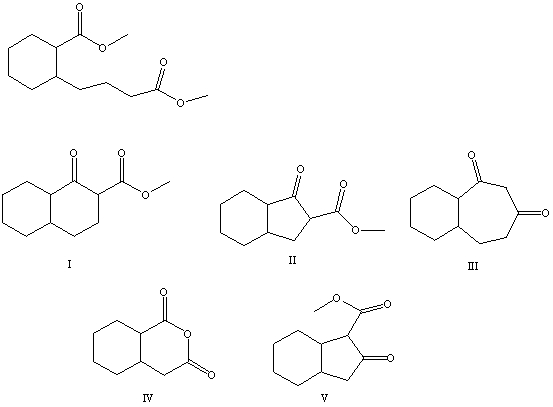
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
9
Which compound could be prepared via Dieckmann condensation? 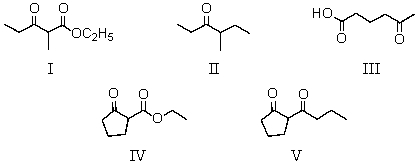
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
10
The Claisen condensation produces which of these?
A) An -keto ester
B) A -keto ester
C) A -hydroxy ester
D) A -hydroxyaldehyde
E) A -diketone
A) An -keto ester
B) A -keto ester
C) A -hydroxy ester
D) A -hydroxyaldehyde
E) A -diketone

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following would afford the best synthesis of diethyl phenylmalonate, 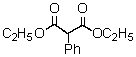
A)
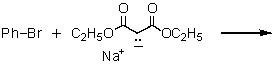
B)
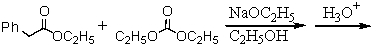
C)
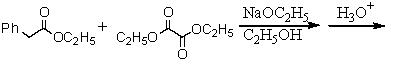
D)
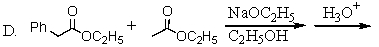
E)
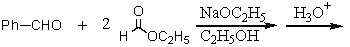

A)
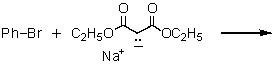
B)
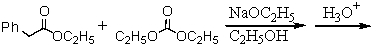
C)

D)
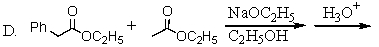
E)
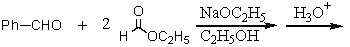

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
12
The reaction of diethyl heptanedioate with sodium ethoxide would give as the product: 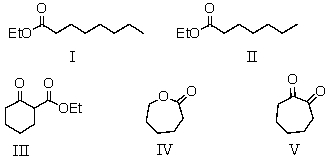
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
13
The reaction of diethyl hexanedioate with sodium ethoxide would give as the product: 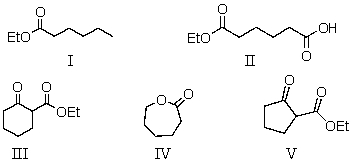
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
14
What product(s)is (are)likely to be obtained upon Dieckmann condensation of the following substance? 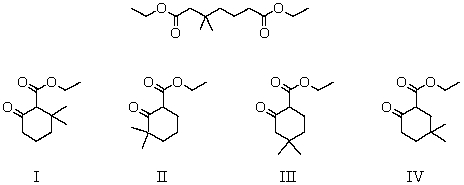
A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV

A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
15
What is the expected product from the following reaction sequence? 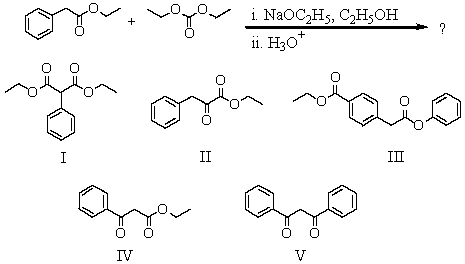
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
16
What product is formed during the following reaction? 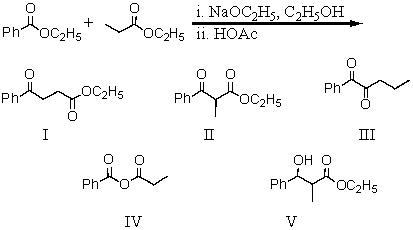
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
17
What would be the major product of the following reaction? 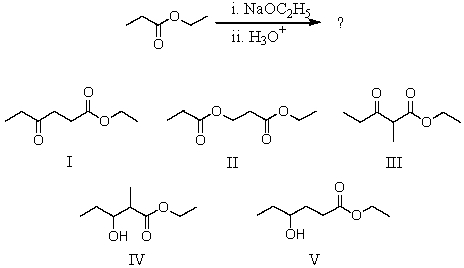
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
18
What is the product of the Dieckmann condensation of this diester, 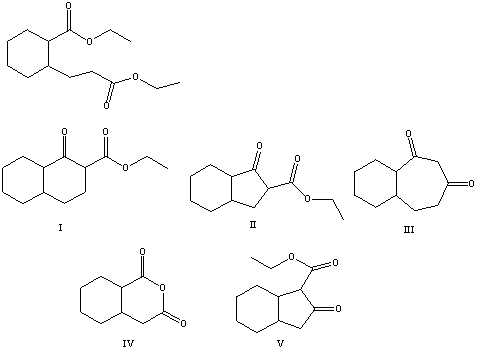
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these combinations is not one which would result in the formation of essentially one Claisen condensation product when one compound is added slowly to the mixture of the other and the base employed?
A)
HCO2Et + CH3CH2CO2Et
B) PhCO2Et + CH3CO2Et
C) (CO2Et)2 + PhCH2CO2Et
D) (CH3)3CCO2Et + CH3CO2Et
E) PhCH2CO2Et + CH3CO2Et
A)
HCO2Et + CH3CH2CO2Et
B) PhCO2Et + CH3CO2Et
C) (CO2Et)2 + PhCH2CO2Et
D) (CH3)3CCO2Et + CH3CO2Et
E) PhCH2CO2Et + CH3CO2Et

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
20
Cyclization reactions,such as the Dieckmann condensation,are best carried out using fairly dilute solutions of the compound to be cyclized.Why is this so?
A) It then is possible to use less base.
B) The reagents generally are expensive.
C) A smaller amount of the compound to be cyclized can be used.
D) Intermolecular condensation is minimized at low concentration.
E) The concentration factor is unimportant.
A) It then is possible to use less base.
B) The reagents generally are expensive.
C) A smaller amount of the compound to be cyclized can be used.
D) Intermolecular condensation is minimized at low concentration.
E) The concentration factor is unimportant.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
21
Which reagents would you use to prepare the following substance from ethyl acetoacetate? 
A) (i)NaH/DMSO;(ii)PhCOCl;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat
B) (i)NaOEt/EtOH;(ii)PhCOCH2Br;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat
C) i)heat;ii)NaOEt/EtOH;iii)PhCOCH2Br
D) (i)NaOEt/EtOH;(ii)PhCl
E) (i)NaOEt/EtOH;(ii)PhCOCl;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat

A) (i)NaH/DMSO;(ii)PhCOCl;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat
B) (i)NaOEt/EtOH;(ii)PhCOCH2Br;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat
C) i)heat;ii)NaOEt/EtOH;iii)PhCOCH2Br
D) (i)NaOEt/EtOH;(ii)PhCl
E) (i)NaOEt/EtOH;(ii)PhCOCl;(iii)OH - /H2O,heat;(iv)H3O+;(v)heat

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
22
What is the product of the Dieckmann-like condensation of this ketoester, 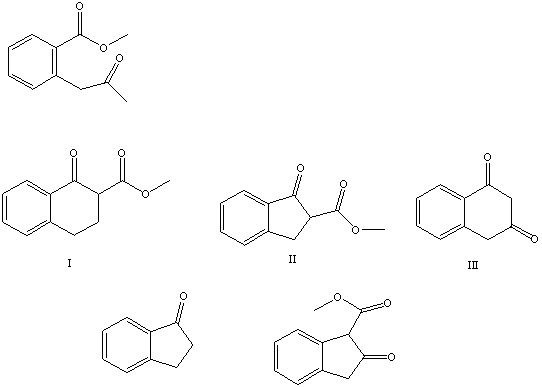 IV V
IV V
A) I
B) II
C) III
D) IV
E) V
 IV V
IV VA) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
23
The product,C,of the following sequence of reactions, 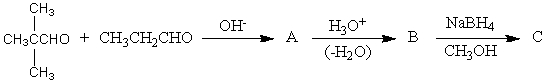 would be:
would be:
A)
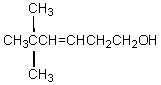
B)
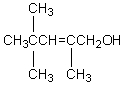
C)
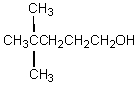
D)
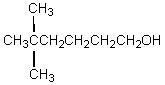
E)
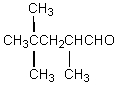
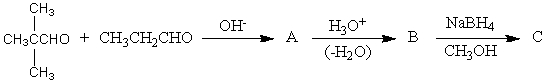 would be:
would be:A)
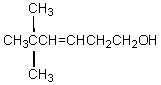
B)

C)
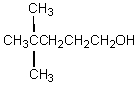
D)
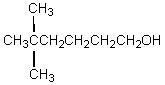
E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
24
Which of these compounds cannot self-condense in the presence of dilute aqueous alkali?
A) 3-(4-Nitrophenyl)propanal
B) 2-Methyl-3-pentanone
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) All of the above compounds can self-condense.
A) 3-(4-Nitrophenyl)propanal
B) 2-Methyl-3-pentanone
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) All of the above compounds can self-condense.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
25
The aldol reaction of cyclohexanone produces which of these self-condensation products? 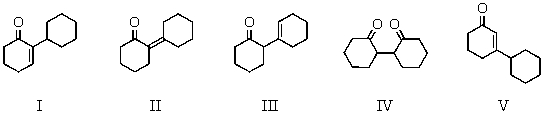
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
26
What would be the major product of the following reaction? 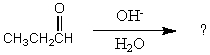
A) CH3CH2CH2OH + CH3CH2COO-
B)
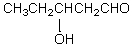
C)
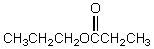
D)
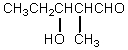
E)
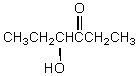
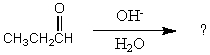
A) CH3CH2CH2OH + CH3CH2COO-
B)
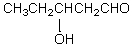
C)
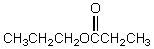
D)
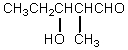
E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
27
Predict the product from the following sequence: 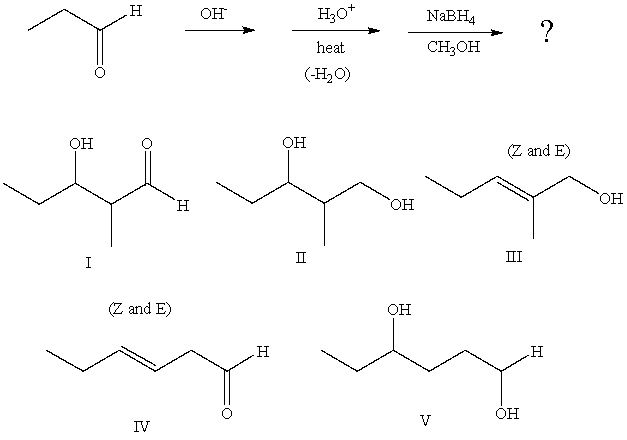
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
28
What would be the major product of the following reaction? 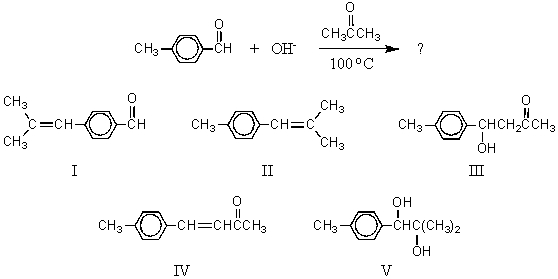
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
29
Predict the product from the following sequence: 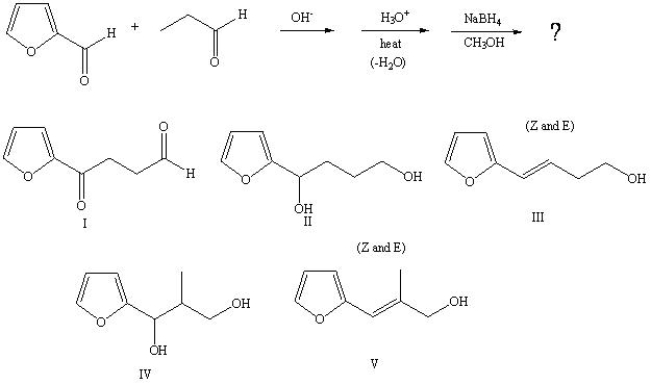
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
30
What is the product of the Dieckmann-like condensation of this ketoester, 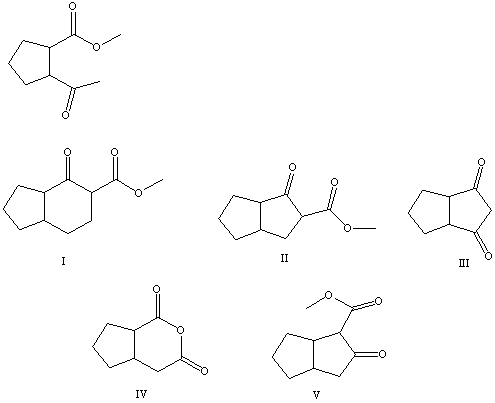
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
31
What would be the major product of the following reaction? 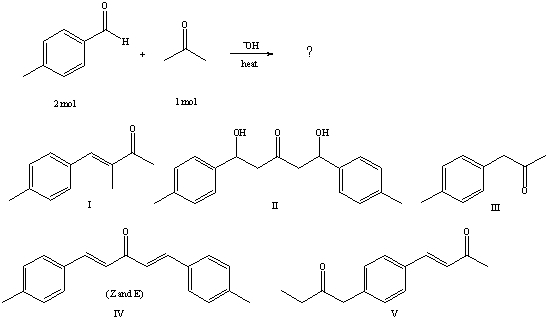
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
32
Predict the product from the following sequence: 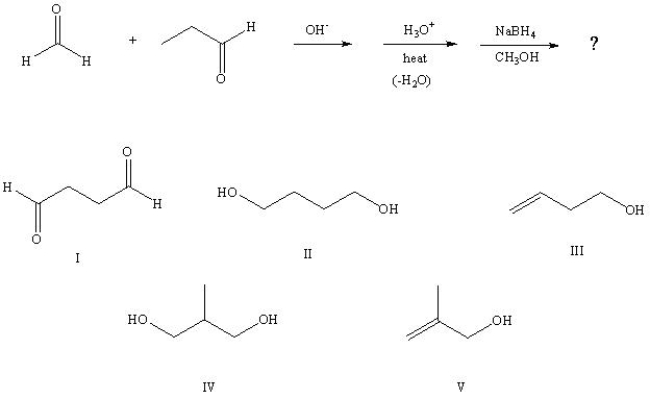
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
33
What product(s)result from the Claisen condensation carried out with an equimolar mixture of ethyl acetate and ethyl propanoate? 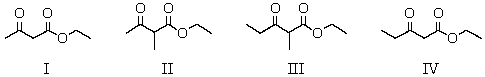
A) I
B) II
C) III
D) IV
E) All of these

A) I
B) II
C) III
D) IV
E) All of these

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
34
The aldol reaction of cyclohexanone produces which of these self-condensation products? 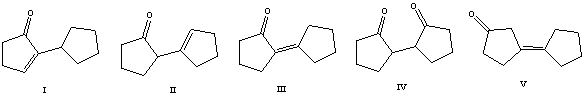
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
35
Which of these is not a reversible process?
A) Base-promoted ester hydrolysis
B) Acid-catalyzed ester hydrolysis
C) Aldol addition
D) Claisen condensation
E) Acetal formation
A) Base-promoted ester hydrolysis
B) Acid-catalyzed ester hydrolysis
C) Aldol addition
D) Claisen condensation
E) Acetal formation

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
36
What is the product of the Dieckmann-like condensation of this ketoester, 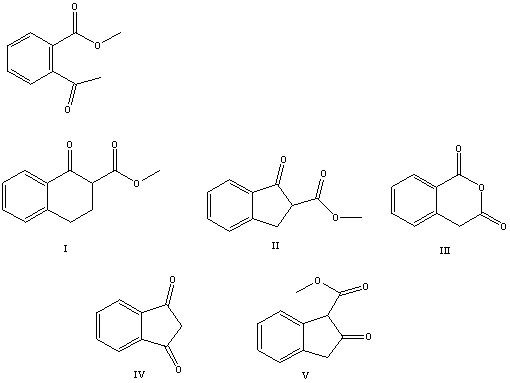
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the only one of these compounds which cannot self-condense in the presence of dilute aqueous alkali?
A) Phenylethanal
B) Propanal
C) 2-Methylpropanal
D) 3-Methylpentanal
E) 2,2-Dimethylpropanal
A) Phenylethanal
B) Propanal
C) 2-Methylpropanal
D) 3-Methylpentanal
E) 2,2-Dimethylpropanal

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
38
What is the product of the Dieckmann-like condensation of this ketoester, 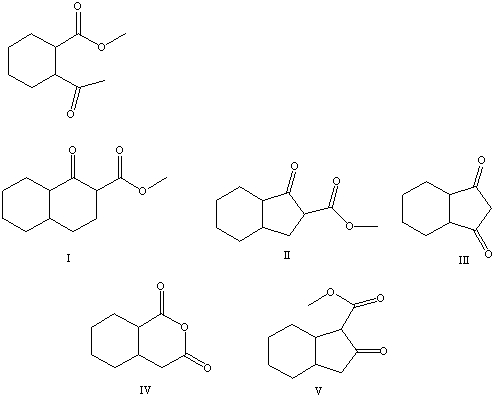
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
39
What would be the major product of the following reaction? 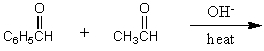
A)
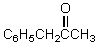
B)
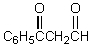
C)
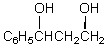
D)
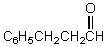
E)
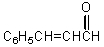

A)
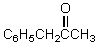
B)
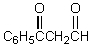
C)
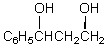
D)
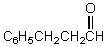
E)
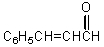

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
40
The aldol condensation product formed from 3-pentanone in the presence of base has the IUPAC name:
A) 5-Ethyl-4-methyl-4-hepten-3-one
B) 5-Ethyl-4-methyl-5-hepten-3-one
C) 4-Methyl-4-nonen-3,7-dione
D) 3-Ethyl-4-methyl-3-hepten-5-one
E) 3-Ethyl-4-methyl-2-hepten-5-one
A) 5-Ethyl-4-methyl-4-hepten-3-one
B) 5-Ethyl-4-methyl-5-hepten-3-one
C) 4-Methyl-4-nonen-3,7-dione
D) 3-Ethyl-4-methyl-3-hepten-5-one
E) 3-Ethyl-4-methyl-2-hepten-5-one

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
41
What would be the final product of the following reaction sequence? 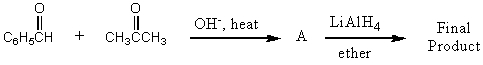
A) C6H5CH2CH2CH2CH3
B)
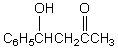
C)
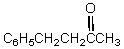
D)
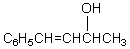
E)
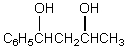
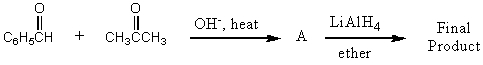
A) C6H5CH2CH2CH2CH3
B)
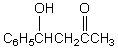
C)
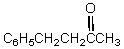
D)
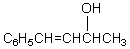
E)
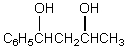

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
42
What would be the major product of the following reaction? 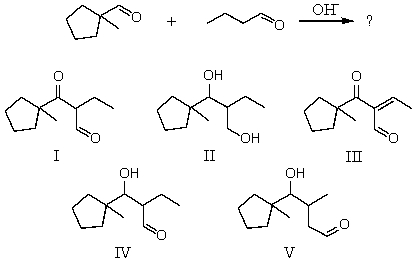
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
43
Which reagents would you use to synthesize this compound by an aldol condensation? 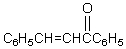
A)
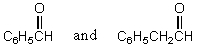
B)
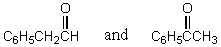
C)
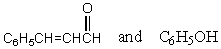
D)
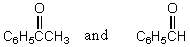
E)
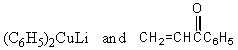
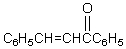
A)
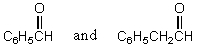
B)
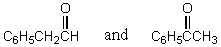
C)
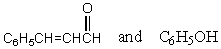
D)
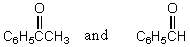
E)
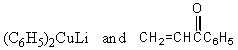

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
44
What would be the major product,B,of the following reaction sequence? 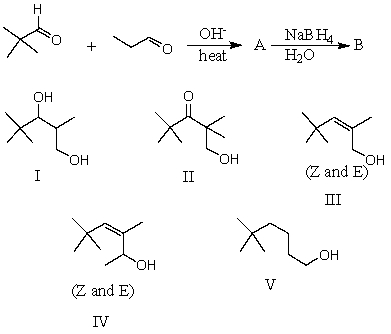
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these compounds can react with 4-methylhexanal to afford good yields of the crossed aldol product?
A) 3-(4-Nitrophenyl)propanal
B) 2-Methyl-3-pentanone
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) None of the above compounds will give good yields of the crossed aldol product with 4-methylhexanal.
A) 3-(4-Nitrophenyl)propanal
B) 2-Methyl-3-pentanone
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) None of the above compounds will give good yields of the crossed aldol product with 4-methylhexanal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
46
The retro-aldol reaction of 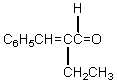 gives:
gives:
A)
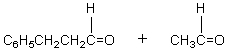
B)
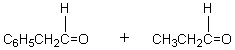
C)
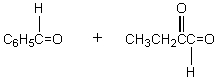
D)
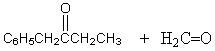
E)
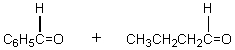
 gives:
gives:A)
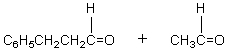
B)
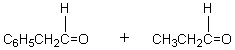
C)
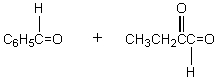
D)
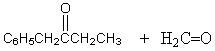
E)
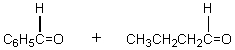

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
47
What would be the product,B,of the following reaction sequence? 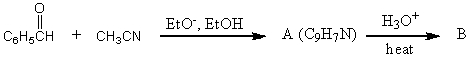
A) C6H5CH=CHCO2H
B)
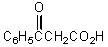
C) C6H5CH2CH2CO2H
D)
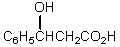
E)
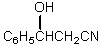

A) C6H5CH=CHCO2H
B)
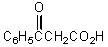
C) C6H5CH2CH2CO2H
D)
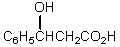
E)
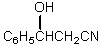

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
48
What would be the major product of the following reaction? 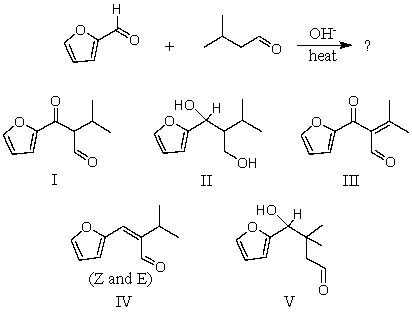
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
49
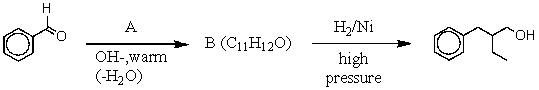
Consider the synthesis above in answering this question.What is compound A?
A) Butanone
B) Butanal
C) Propanal
D) 1-Butanol
E) 2-Methylpropanal

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
50
What would be the major product of the following reaction? 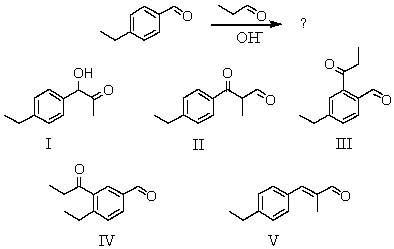
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
51
What would be the major product,B,of the following reaction sequence? 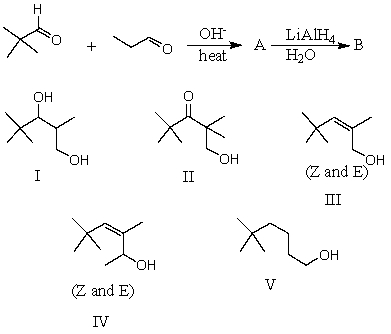
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
52
Which of these is not among the reaction products when a crossed aldol addition occurs between ethanal and butanal?
A)
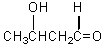
B)
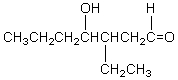
C)
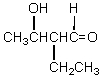
D)
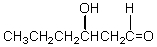
E)
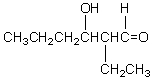
A)
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
53
What would be the major product,B,of the following reaction sequence? 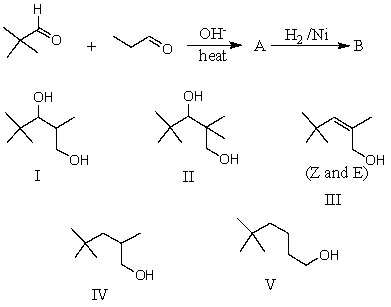
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
54
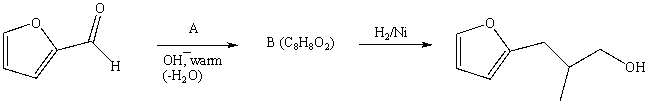
What is the intermediate B in the synthesis shown above?
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
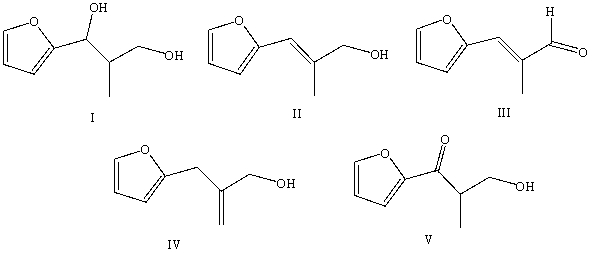
55
What would be the product,C,of the following reaction sequence? 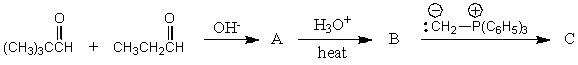
A) (CH3)3CCH2CH2CH2OH
B)
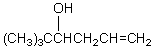
C)
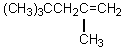
D)
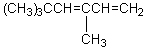
E)
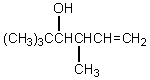
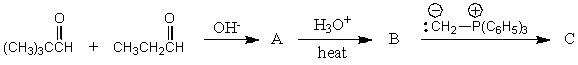
A) (CH3)3CCH2CH2CH2OH
B)
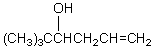
C)
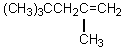
D)

E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
56
What would be the product,C,of the following reaction sequence? 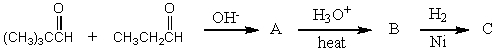
A) (CH3)3CCH2CH2CH2OH
B)
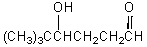
C)
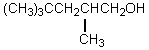
D)
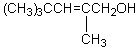
E)
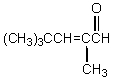
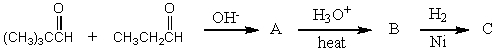
A) (CH3)3CCH2CH2CH2OH
B)
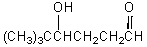
C)
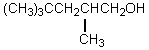
D)
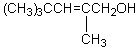
E)
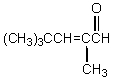

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
57
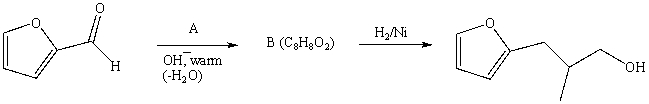
Consider the synthesis above in answering this question.What is compound A?
A) Butanone
B) Butanal
C) Propanal
D) 1-Butanol
E) 2-Methylpropanal

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
58
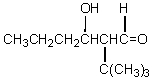
If butanal is added slowly to an aqueous solution of sodium hydroxide and 2,2-dimethylpropanal at 25 C,the principal product is which of these?
A)
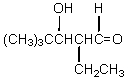
B)
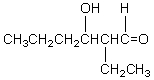
C)
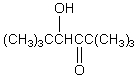
D)
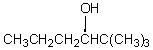
E)
A)

B)

C)
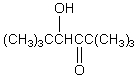
D)
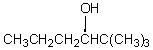
E)


Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
59
Which of these compounds can react with 4-methylpentanal to afford good yields of the crossed aldol product?
A) 3-(4-Nitrophenyl)propanal
B) 2-Ethyl-2-methylheptanal
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) None of the above compounds will give good yields of the crossed aldol product with 4-methylhexanal.
A) 3-(4-Nitrophenyl)propanal
B) 2-Ethyl-2-methylheptanal
C) 2-(4-Nitrophenyl)propanal
D) 3-(4-Nitrophenyl)-2-butanone
E) None of the above compounds will give good yields of the crossed aldol product with 4-methylhexanal.

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
60
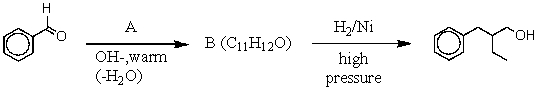
What is the intermediate B in the synthesis shown above?
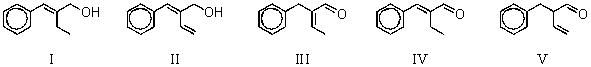
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
61
When 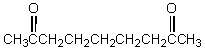 cyclizes in basic solution,which of these compounds will be formed?
cyclizes in basic solution,which of these compounds will be formed? 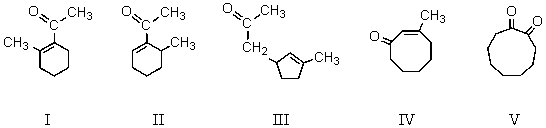
A) I
B) II
C) III
D) IV
E) V
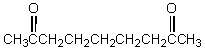 cyclizes in basic solution,which of these compounds will be formed?
cyclizes in basic solution,which of these compounds will be formed? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
62
What product results from the intramolecular aldol reaction of 2,5-hexanedione? 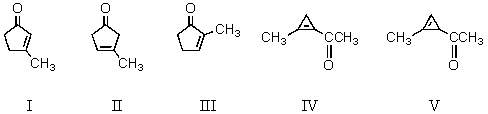
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
63
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 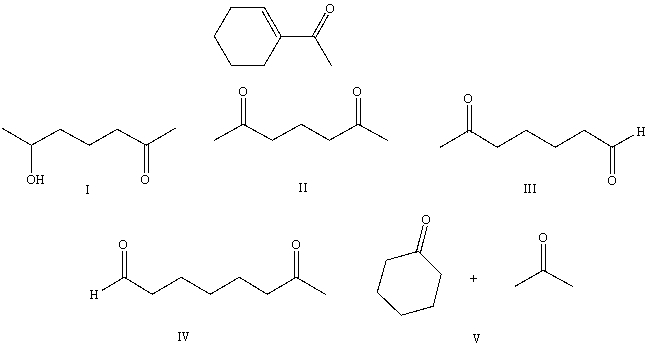
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
64
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 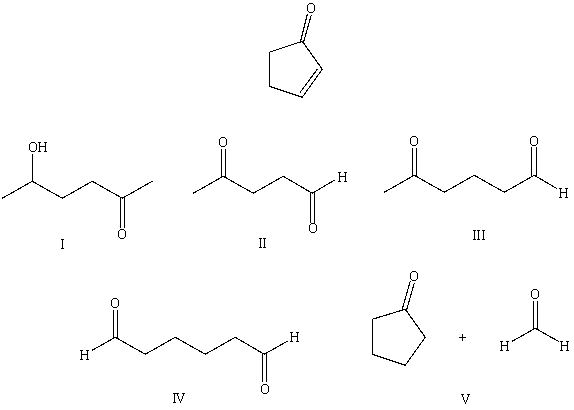
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
65
What is the missing reagent? 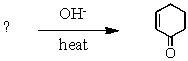
A)
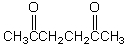
B)
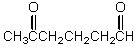
C)
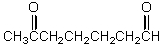
D)
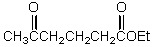
E)
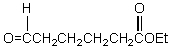

A)
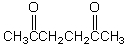
B)
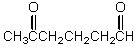
C)
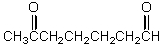
D)
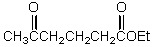
E)
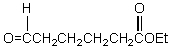

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
66
What is the major product of the following reaction? 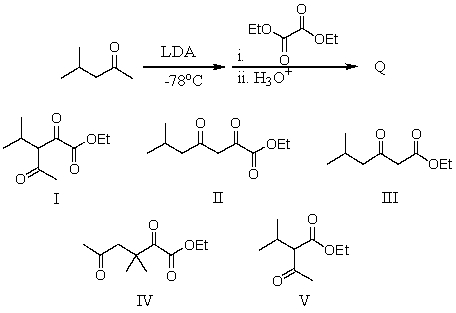
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
67
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 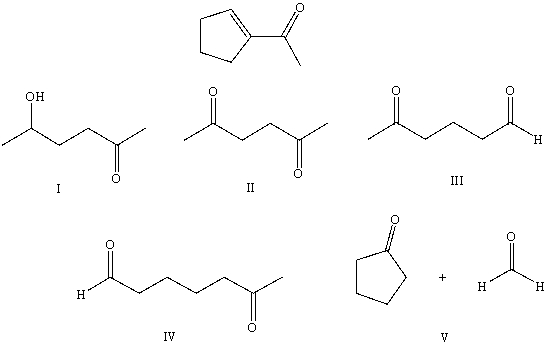
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
68
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 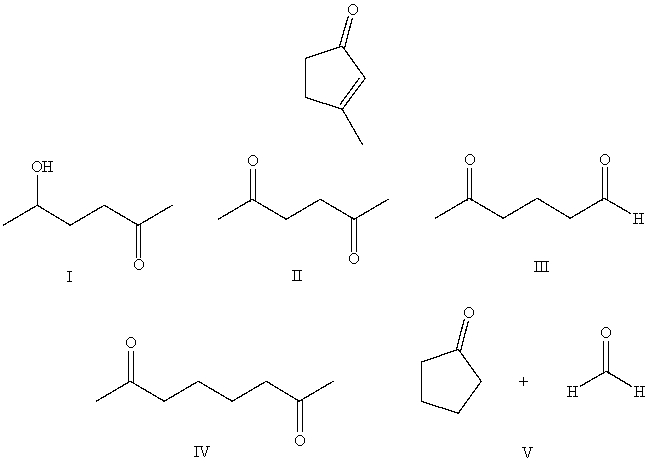
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
69
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 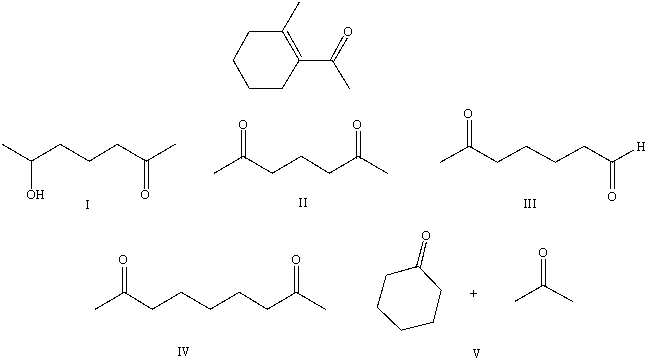
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction of 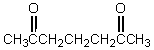 with base affords which of these products?
with base affords which of these products? 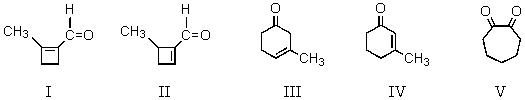
A) I
B) II
C) III
D) IV
E) V
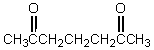 with base affords which of these products?
with base affords which of these products? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
71
What would be the product,C,of the following reaction sequence? 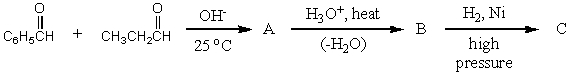
A)
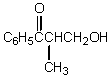
B)
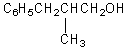
C)
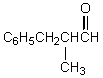
D)
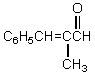
E)
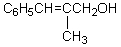
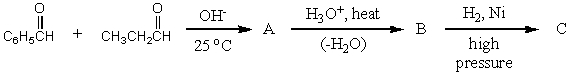
A)

B)
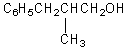
C)
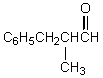
D)
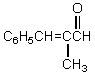
E)
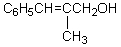

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
72
What compound results from the aldol cyclization of 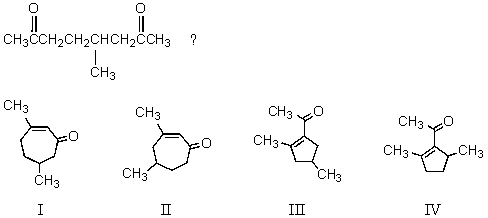
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
73
What product is formed during the following reaction? 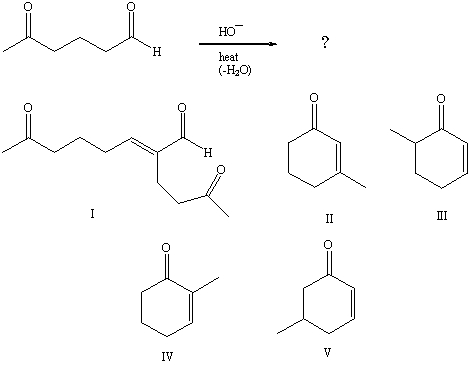
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
74
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 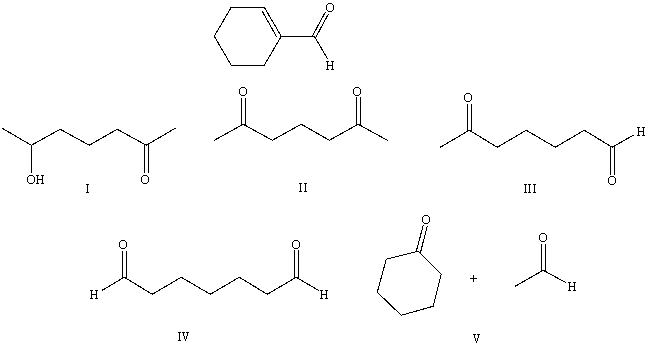
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
75
The product of the following reaction is: 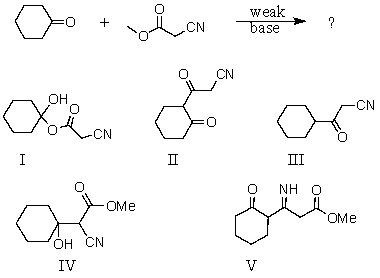
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
76
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 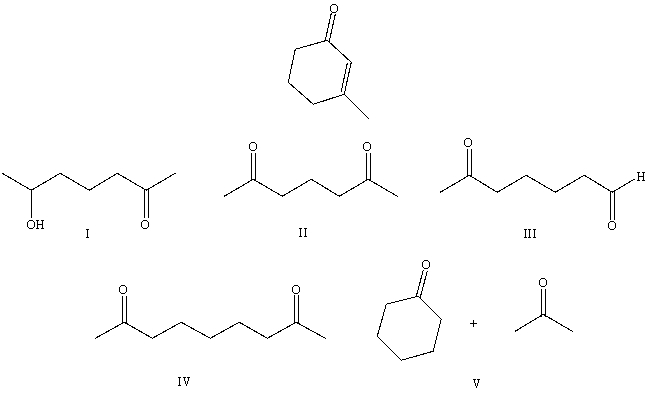
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
77
What starting compound(s)would you use in an aldol reaction to prepare 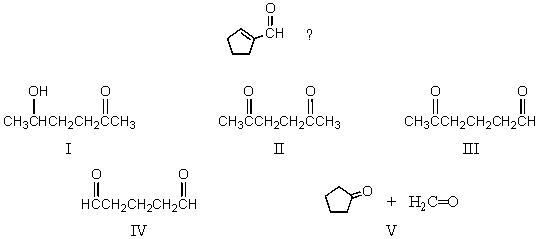
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
78
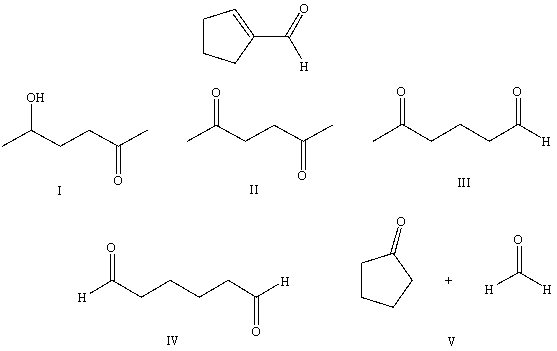
The aldol cyclization of  produces which of these?
produces which of these? 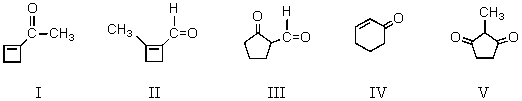
A) I
B) II
C) III
D) IV
E) V
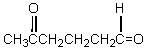 produces which of these?
produces which of these? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
79
What starting compound(s)would you use in an aldol reaction to prepare as the major product: 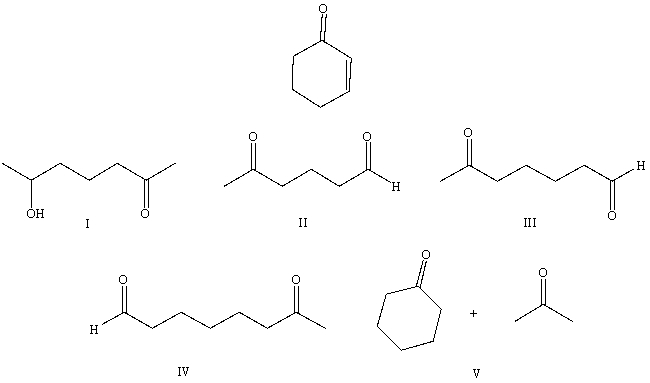
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck
80
What would be the product,C,of the following reaction sequence? 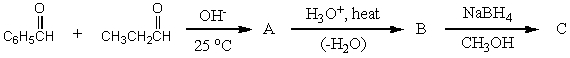
A)
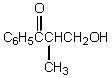
B)
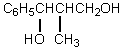
C)
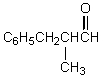
D)
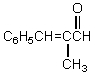
E)
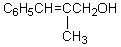 hi
hi
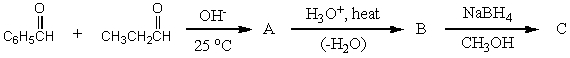
A)

B)
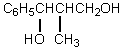
C)
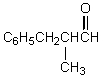
D)
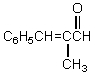
E)
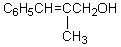 hi
hi
Unlock Deck
Unlock for access to all 131 flashcards in this deck.
Unlock Deck
k this deck


