Deck 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 21: Phenols and Aryl Halides: Nucleophilic Aromatic Substitution
1
What is the common name for 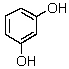
A) m-Hydroxyphenol
B) m-Dihydroxybenzene
C) Resorcinol
D) 1,3-dihydroxybenzene
E) 1,3-Benzenediol

A) m-Hydroxyphenol
B) m-Dihydroxybenzene
C) Resorcinol
D) 1,3-dihydroxybenzene
E) 1,3-Benzenediol
Resorcinol
2
What would be the major product of the following reaction? 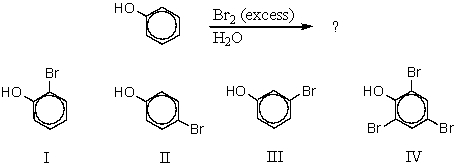
A) I
B) II
C) III
D) IV
E) A mixture of I and II

A) I
B) II
C) III
D) IV
E) A mixture of I and II
IV
3
Which of the following phenols would have the largest pKa? 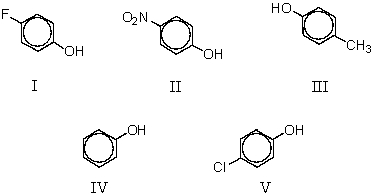
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
III
4
What is the IUPAC name for 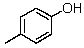
A) p-Hydroxyphenol
B) p-Dihydroxybenzene
C) Resorcinol
D) 1,4-dihydroxybenzene
E) 4-methylphenol

A) p-Hydroxyphenol
B) p-Dihydroxybenzene
C) Resorcinol
D) 1,4-dihydroxybenzene
E) 4-methylphenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Which products would be formed in the following reaction? 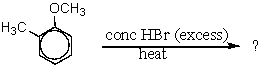
A) Methoxybenzene + methyl bromide
B) 2-Methylphenol + methyl bromide
C) 2-Bromotoluene + methanol
D) 2-Bromotoluene + methyl bromide
E) Bromobenzene + methyl bromide
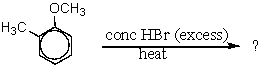
A) Methoxybenzene + methyl bromide
B) 2-Methylphenol + methyl bromide
C) 2-Bromotoluene + methanol
D) 2-Bromotoluene + methyl bromide
E) Bromobenzene + methyl bromide

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
Which of these is an acceptable synthesis of phenetole (ethyl phenyl ether)?
A)
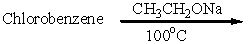
B)
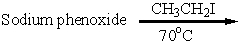
C)
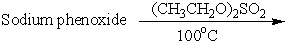
D)
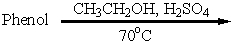
E) A and C
A)
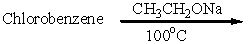
B)
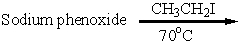
C)
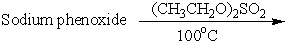
D)
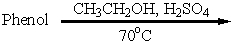
E) A and C

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would be the strongest acid? 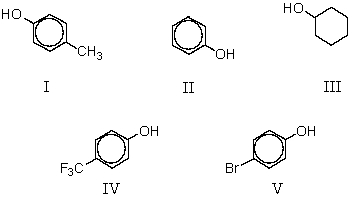
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
When sodium phenoxide is heated to 125 C with carbon dioxide under pressure and the product mixture acidified,which of these is produced? 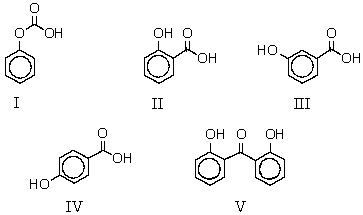
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
What are the products of the reaction of phenol with propanoic anhydride in the presence of base? 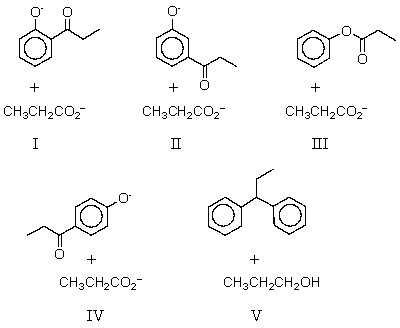
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
The common name for 1,2-benzenediol is which of these?
A) Catechol
B) m -Cresol
C) Resorcinol
D) Hydroquinone
E) o-Xylenol
A) Catechol
B) m -Cresol
C) Resorcinol
D) Hydroquinone
E) o-Xylenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name for 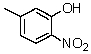
A) m-Hydroxy-p-nitrotoluene
B) 3-Hydroxy-4-nitrotoluene
C) 5-methyl-2-nitrophenol
D) 3-methyl-2-nitrophenol
E) 2-Hydroxy-4methylnitrobenzene

A) m-Hydroxy-p-nitrotoluene
B) 3-Hydroxy-4-nitrotoluene
C) 5-methyl-2-nitrophenol
D) 3-methyl-2-nitrophenol
E) 2-Hydroxy-4methylnitrobenzene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
The common name for 3-methylphenol is which of these?
A) Catechol
B) m -Cresol
C) m-tolylol
D) Hydroquinone
E) p-Xylenol
A) Catechol
B) m -Cresol
C) m-tolylol
D) Hydroquinone
E) p-Xylenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Refluxing anisole,CH3OC6H5,with excess concentrated HBr would yield which of these product mixtures?
A) C6H5Br + CH3OH
B) C6H5OH + CH4
C) C6H5OH + CH3OH
D) C6H5Br + CH3Br
E) C6H5OH + CH3Br
A) C6H5Br + CH3OH
B) C6H5OH + CH4
C) C6H5OH + CH3OH
D) C6H5Br + CH3Br
E) C6H5OH + CH3Br

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound would be most acidic?
A) Cyclohexanol
B) 1-Hexanol
C) Phenol
D) 4-Methylphenol
E) 4-Chlorophenol
A) Cyclohexanol
B) 1-Hexanol
C) Phenol
D) 4-Methylphenol
E) 4-Chlorophenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Indicate the correct product,if any,of the following reaction. 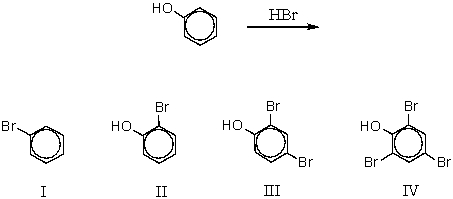
A) I
B) II
C) III
D) IV
E) There is no net reaction.

A) I
B) II
C) III
D) IV
E) There is no net reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these species is the strongest base? 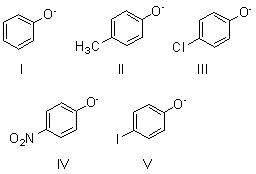
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds would you expect to be the strongest acid?
A) CH3OH
B) C6H5CH2OH
C) p-CH3C6H4OH
D) C6H5OH
E) p-NO2C6H4OH
A) CH3OH
B) C6H5CH2OH
C) p-CH3C6H4OH
D) C6H5OH
E) p-NO2C6H4OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
What is the product of the reaction of phenol and chloroacetic acid in basic solution,followed by acidification? 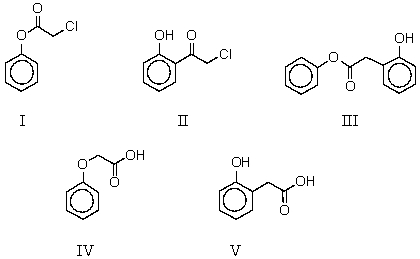
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
Which method could be used for preparing methoxybenzene from phenol?
A) NaOH,then CH3I
B) NaOH,then CH3OSO3CH3
C) NaOH,then CH3OCH3
D) A and B
E) All of these
A) NaOH,then CH3I
B) NaOH,then CH3OSO3CH3
C) NaOH,then CH3OCH3
D) A and B
E) All of these

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
The common name for 4-methylphenol is which of these?
A) Catechol
B) p-Cresol
C) Resorcinol
D) Hydroquinone
E) p-Xylenol
A) Catechol
B) p-Cresol
C) Resorcinol
D) Hydroquinone
E) p-Xylenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
What is the product of the following synthesis? 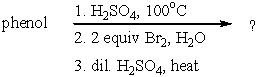
A) 2,3-Dibromophenol
B) 2,4-Dibromophenol
C) 2,6-Dibromophenol
D) 2-Hydroxy-3,5-dibromobenzenesulfonic acid
E) 2,4,6-Tribromophenol
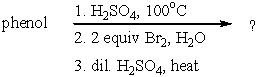
A) 2,3-Dibromophenol
B) 2,4-Dibromophenol
C) 2,6-Dibromophenol
D) 2-Hydroxy-3,5-dibromobenzenesulfonic acid
E) 2,4,6-Tribromophenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
What product is likely to be obtained by the action of Ag+ or Fe+3 on the following substance? 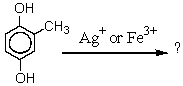
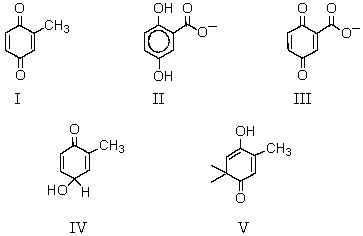
A) I
B) II
C) III
D) IV
E) V
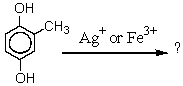

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
Predict the product of this Cope rearrangement: 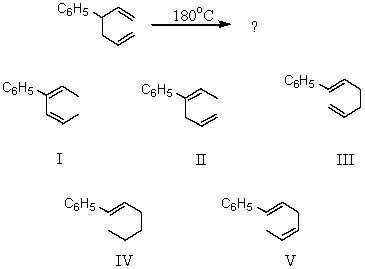
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following would provide a synthesis of aspirin, o-CH3COOC6H4COOH?
A) C6H5COOH,CH3COOH,AlCl3,heat;then H2O
B) CH3COOC6H5,CO2,heat;then H3O+
C) CH3COOC6H5,HCOOC2H5,C2H5O-;then H3O+;then OH-
D) C6H5OH,CO2,H3O+;separate isomers;then CH3COOH,AlCl3
E) C6H5OH,OH-,CO2,heat,pressure;then H3O+;then (CH3CO)2O
A) C6H5COOH,CH3COOH,AlCl3,heat;then H2O
B) CH3COOC6H5,CO2,heat;then H3O+
C) CH3COOC6H5,HCOOC2H5,C2H5O-;then H3O+;then OH-
D) C6H5OH,CO2,H3O+;separate isomers;then CH3COOH,AlCl3
E) C6H5OH,OH-,CO2,heat,pressure;then H3O+;then (CH3CO)2O

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
Which of these reactions does not produce phenol? I think this question is ok
A)
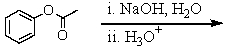
B)
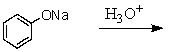
C)
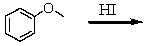
D)
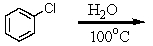
E)
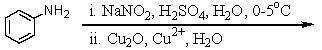
A)
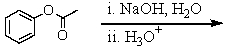
B)
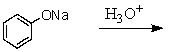
C)
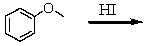
D)
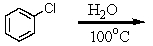
E)
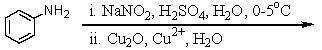

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
What is the product of the reaction of 1 mol of p-benzoquinone with 1 mol of isoprene (2-methyl-1,3-butadiene)? 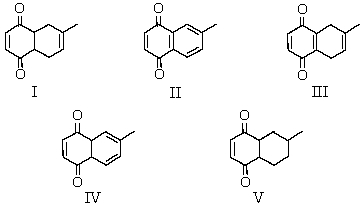
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
Thymol,isolated from thyme,has the following structure: 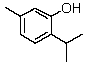 Which of the following types of signal is NOT expected to be observed in its NMR spectrum?
Which of the following types of signal is NOT expected to be observed in its NMR spectrum?
A) singlet
B) doublet
C) triplet
D) septet
E) all of the above types of signals are expected to be observed
 Which of the following types of signal is NOT expected to be observed in its NMR spectrum?
Which of the following types of signal is NOT expected to be observed in its NMR spectrum?A) singlet
B) doublet
C) triplet
D) septet
E) all of the above types of signals are expected to be observed

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
What products(s)would you expect from the following reaction? 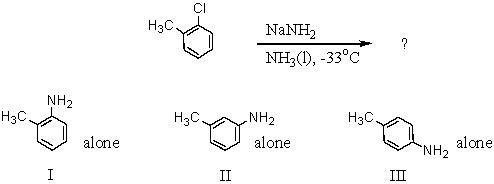
A) I
B) II
C) III
D) Products I and II
E) All of the above

A) I
B) II
C) III
D) Products I and II
E) All of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
What product should be obtained if benzyne is generated in the presence of 1,3-butadiene? 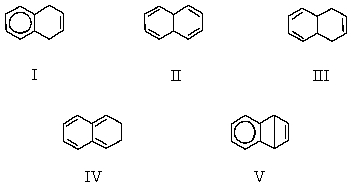
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
In terms of reactivity towards nucleophiles,bromobenzene is most similar to which of these?
A) Allyl bromide
B) Vinyl bromide
C) tert-Butyl bromide
D) Propyl bromide
E) Methyl bromide
A) Allyl bromide
B) Vinyl bromide
C) tert-Butyl bromide
D) Propyl bromide
E) Methyl bromide

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
What is the final product? 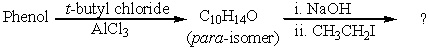
A) 1-tert-Butyl-4-ethoxybenzene
B) 1-tert-Butyl-4-ethylbenzene
C) 1-tert-Butoxy-4-ethoxybenzene
D) tert-Butyl ethyl ether
E) 1-tert-Butoxy-3-ethylbenzene
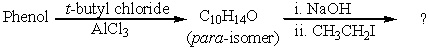
A) 1-tert-Butyl-4-ethoxybenzene
B) 1-tert-Butyl-4-ethylbenzene
C) 1-tert-Butoxy-4-ethoxybenzene
D) tert-Butyl ethyl ether
E) 1-tert-Butoxy-3-ethylbenzene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
Which is the leaving group when the following substance reacts with sodium cyanide in DMSO solution? 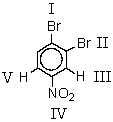
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
Which position is predicted to be the chief site of substitution when the following substance reacts with bromine in carbon disulfide at 10 C ? 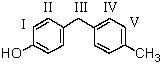
A) I
B) II
C) III
D) IV
E) V
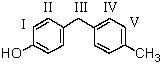
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would be most likely to undergo a nucleophilic substitution reaction with aqueous sodium hydroxide by an addition-elimination mechanism? 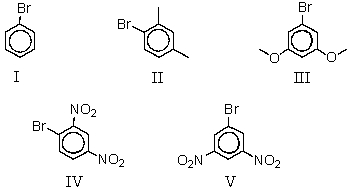
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following reactions would yield p-tert-butylphenol?
A)
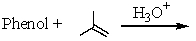
B)
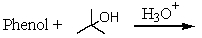
C)
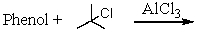
D) All of these
E) None of these
A)

B)

C)
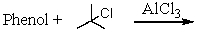
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
The formation of equal amounts of m-toluidine (m-aminotoluene)and p-toluidine in the reaction of p-bromotoluene with sodium amide in liquid ammonia at -33 C suggests this species as the reaction intermediate: 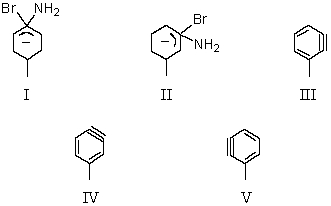
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Which product is likely to be obtained during the following reaction of the isotope-labeled allyl ether (14C-isotopic site marked with an asterisk)? 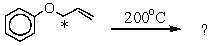
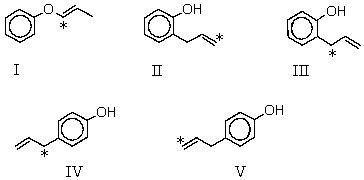
A) I
B) II
C) III
D) IV
E) V
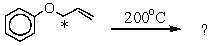

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
What is the major product of the following reaction? 
A) p-Hydroxybenzenesulfonic acid
B) p-Nitrophenol
C) o-Nitrophenol
D) B and C
E) A and B
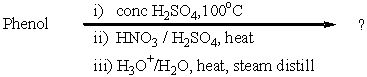
A) p-Hydroxybenzenesulfonic acid
B) p-Nitrophenol
C) o-Nitrophenol
D) B and C
E) A and B

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these resonance structures makes the greatest contribution to the hybrid for the intermediate in the SNAr reaction of o-chloronitrobenzene with methoxide ion? 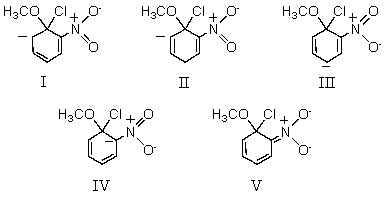
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
What product(s)would you expect from the following reaction? 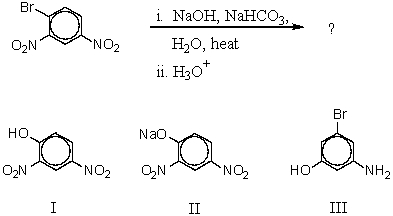
A) I
B) II
C) III
D) Substantial amounts of I and II
E) Substantial amounts of I,II,and III

A) I
B) II
C) III
D) Substantial amounts of I and II
E) Substantial amounts of I,II,and III

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
Most of the worldwide industrial preparation of phenol is now based on the ___________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
The cumene hydroperoxide synthesis of phenol is industrially very satisfying,because it takes two inexpensive starting materials,___________ and ___________,and converts them into two valuable products,phenol and __________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
The industrial synthesis of phenol from cumene involves the acid hydrolysis of the key intermediate,cumene hydroperoxide (formed from cumene via oxidation).Outline the mechanistic steps that lead to this oxidation product.Which other industrially important substance is produced as a by-product in this reaction? 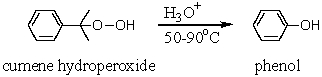
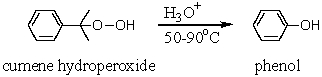

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Outline the steps involved in the synthesis of 3-nitrophenol from 1,3-dinitrobenzene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Outline the steps involved in the synthesis of 3-nitrophenol from benzoic acid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Methyl salicylate,commonly found in topical muscle relaxants,has the following structure: 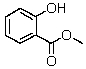 Which of the following signals is NOT expected to be observed in its NMR spectrum?
Which of the following signals is NOT expected to be observed in its NMR spectrum?
A) Singlet at ~4 ppm (3H)
B) Doublet at ~7 ppm (1H)
C) Quartet at about 7 ppm (4H)
D) Singlet at ~ 6 ppm (1H)
E) Two of the above types of signals are not expected to be observed
 Which of the following signals is NOT expected to be observed in its NMR spectrum?
Which of the following signals is NOT expected to be observed in its NMR spectrum?A) Singlet at ~4 ppm (3H)
B) Doublet at ~7 ppm (1H)
C) Quartet at about 7 ppm (4H)
D) Singlet at ~ 6 ppm (1H)
E) Two of the above types of signals are not expected to be observed

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the structure corresponding to the following IUPAC name:
4-benzyl-3,5-diiodophenol
4-benzyl-3,5-diiodophenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the structure corresponding to the following IUPAC name:
(2E,4S)-4-(3-hydroxyphenyl)-2-pentenoic acid
(2E,4S)-4-(3-hydroxyphenyl)-2-pentenoic acid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
What is the effect of electron releasing and withdrawing group on ring acidity?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
Compound L has the molecular formula C11H16O.L is insoluble in water but dissolves in aqueous NaOH.The infrared spectrum of L shows a broad absorption band in the 3200-3600 cm-1 region;its 1H NMR spectrum consists of: triplet, δ 0.80 (3H)
Singlet, δ1.2 (6H)
Quartet,δ 1.5 (2H)
Singlet, δ4.5 (1H)
Multiplet,δ 7.0 (4H)
The most likely structure for compound L is: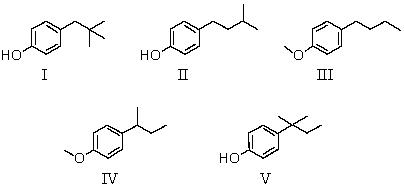
A) I
B) II
C) III
D) IV
E) V
Singlet, δ1.2 (6H)
Quartet,δ 1.5 (2H)
Singlet, δ4.5 (1H)
Multiplet,δ 7.0 (4H)
The most likely structure for compound L is:

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Propose three synthetic methods to outline the steps that could be used (showing all
reagents and reaction conditions)to carry out the transformation shown below.
reagents and reaction conditions)to carry out the transformation shown below.


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
The spectra shown below are for the compound A.Explain each spectrum with labeling all
the peaks and Identify compounds A-C.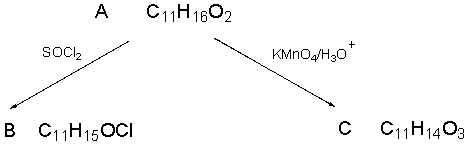
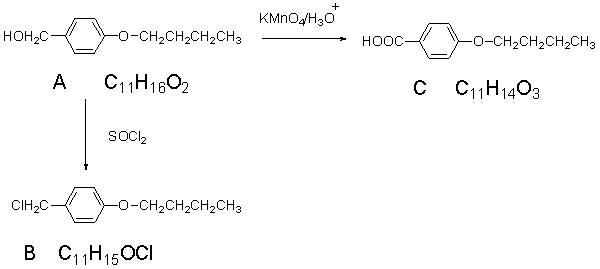 IR Spectrum of Compound A
IR Spectrum of Compound A 
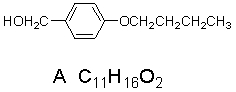 1HNMR Spectrum of Compound A
1HNMR Spectrum of Compound A 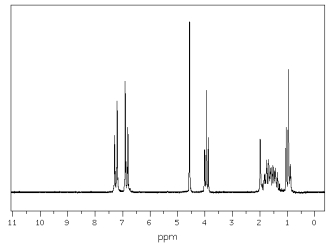
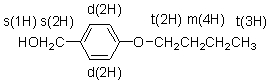 13CNMR Spectrum of Compound A
13CNMR Spectrum of Compound A 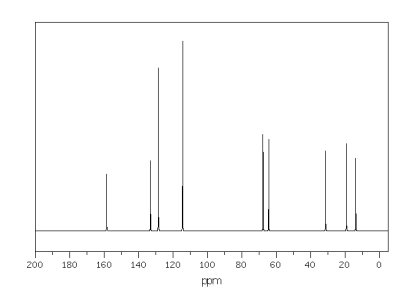 9 different signals = 9 different protons
9 different signals = 9 different protons
the peaks and Identify compounds A-C.

 IR Spectrum of Compound A
IR Spectrum of Compound A 
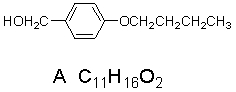 1HNMR Spectrum of Compound A
1HNMR Spectrum of Compound A 
 13CNMR Spectrum of Compound A
13CNMR Spectrum of Compound A  9 different signals = 9 different protons
9 different signals = 9 different protons
Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
Outline the steps involved in the synthesis of 3-bromo-4-methylphenol from 4-nitrotoluene

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
Give the IUPAC name of the following substance: 


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
The most important laboratory synthesis of phenols is by _______________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
Draw the structure corresponding to the following IUPAC name:
(R)-2-Bromo-5-(3-methylpentyl)phenol
(R)-2-Bromo-5-(3-methylpentyl)phenol

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
Eugenol,isolated from cloves,has the following structure: 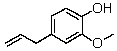 Which of the following signals is NOT expected to be observed in its NMR spectrum?
Which of the following signals is NOT expected to be observed in its NMR spectrum?
A) Singlet at ~4 ppm (3H)
B) Doublet at ~2.5 ppm (2H)
C) Singlet at ~ 7 ppm (1H)
D) Singlet at ~ 6 ppm (1H)
E) All of the above types of signals are expected to be observed
 Which of the following signals is NOT expected to be observed in its NMR spectrum?
Which of the following signals is NOT expected to be observed in its NMR spectrum?A) Singlet at ~4 ppm (3H)
B) Doublet at ~2.5 ppm (2H)
C) Singlet at ~ 7 ppm (1H)
D) Singlet at ~ 6 ppm (1H)
E) All of the above types of signals are expected to be observed

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
The commonly used name for hydroxybenzene is __________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
A key step in the industrial synthesis of phenol from cumene is the oxidation of cumene to cumene hydroperoxide with oxygen.Outline the mechanistic steps that lead to this oxidation product. 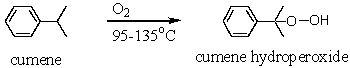
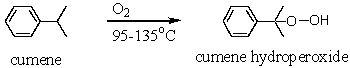

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
Write the IUPAC name of aspirin and its use.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Nitration of phenol gives only ortho and para products.What is the reason for this?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Phenol is an enol.In water it will undergo an enol to keto tautomerization
1)Will this reaction likely go by a base or acid catalyzed mechanism? Show the
mechanism.
2)Which is more stable the enol or ketoform? Why?
1)Will this reaction likely go by a base or acid catalyzed mechanism? Show the
mechanism.
2)Which is more stable the enol or ketoform? Why?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the major products formed by the reaction of phenol with each of the following:
(a)HNO3 / H2SO4 / heat
(b)CH3CH2Cl / AlCl3 / heat
(c)H2SO4 / SO3/ heat
(d)CH3CH2COCl / AlCl3 / heat /H2/Pd-C
(e)Na then add benzyl chloride
(f)HBr
(a)HNO3 / H2SO4 / heat
(b)CH3CH2Cl / AlCl3 / heat
(c)H2SO4 / SO3/ heat
(d)CH3CH2COCl / AlCl3 / heat /H2/Pd-C
(e)Na then add benzyl chloride
(f)HBr

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Explain why cleavage of phenyl alkyl ether with HBr always give phenol and alkyl bromide.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
Draw the structure of the product obtained when benzoquinone reacts with 2 molar equivalents of 1,3-butadiene via a Diels Alder reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
When phenol is nitrated with dilute nitric acid,a mixture of o- and p- substituted products is obtained.These products can then be separated by the technique of steam distillation.However,analogous products from the nitration of methoxybenzene cannot be similarly separated by steam distillation.Explain.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Draw the structure of the product obtained when the following substance is heated strongly: 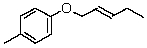


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Draw the structure of the final product obtained when 3-methylphenol is subjected to the following reaction sequence:
i)NaOH;ii)CH3CH2CH2OSO2OCF3
i)NaOH;ii)CH3CH2CH2OSO2OCF3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Draw the structure of the ionic species obtained when the following substance is treated with NaOH: 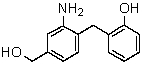


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Discuss the less boiling point of o-nitrophenol compared to p-nitrophenol.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Discuss the following reaction
(a)Williamson's synthesis (b)Kolbe's reaction
(a)Williamson's synthesis (b)Kolbe's reaction

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Draw the structure of the final product obtained when 2-nitrophenol reacts with acetic anhydride in presence of base.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Which is a stronger acid: Phenol or p-cresol?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The Claisen and Cope rearrangements are two examples of a general class of reactions called ___________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Suggest a simple chemical test to distinguish between 4-methylphenol and 2,4,6-trinitrophenol,briefly explaining your rationale.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Draw the structure of the final product obtained when 4-cyclopentylphenol is subjected to the following reaction sequence:
i)NaOH;ii)CH3I;iii)NBS;iv)CH3ONa/CH3OH,heat
i)NaOH;ii)CH3I;iii)NBS;iv)CH3ONa/CH3OH,heat

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
Arrange the following in increasing order of acidity. 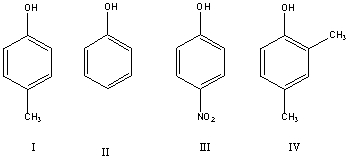


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
Suggest a reasonable synthetic strategy for the synthesis of 3-bromophenol from acetophenone

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
Benzyne is a very unstable intermediate,because _____________.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
Outline the steps involved in the synthesis of p-methoxybenzoic acid from phenol.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


