Deck 11: Alcohols and Ethers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/172
Play
Full screen (f)
Deck 11: Alcohols and Ethers
1
What is the relationship between alcohols I and II? 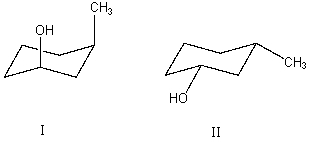 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
enantiomers.
2
A correct IUPAC name for isobutyl alcohol is:
A) 2-Methyl-1-propanol
B) 2-Methyl-1-butanol
C) 1-Methyl-1-propanol
D) 1,1-Dimethyl-1-ethanol
E) 3-Methyl-1-propanol
A) 2-Methyl-1-propanol
B) 2-Methyl-1-butanol
C) 1-Methyl-1-propanol
D) 1,1-Dimethyl-1-ethanol
E) 3-Methyl-1-propanol
2-Methyl-1-propanol
3
What is the relationship between alcohols I and II?  They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
enantiomers.
4
The IUPAC name of compound 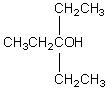 is:
is:
A) 1,1,1-Triethylmethanol
B) 1,1-Diethyl-1-propanol
C) 2-Ethyl-3-pentanol
D) 3-Ethyl-3-pentanol
E) tert-Heptanol
 is:
is:A) 1,1,1-Triethylmethanol
B) 1,1-Diethyl-1-propanol
C) 2-Ethyl-3-pentanol
D) 3-Ethyl-3-pentanol
E) tert-Heptanol

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
5
What is the relationship between alcohols I and II? 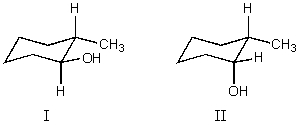 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
6
The correct IUPAC name for tert-butyl alcohol is:
A) 1-Butanol
B) 2-Methyl-1-propanol
C) 2-Methyl-2-propanol
D) 2-Butanol
E) 1,1-Dimethyl-1-ethanol
A) 1-Butanol
B) 2-Methyl-1-propanol
C) 2-Methyl-2-propanol
D) 2-Butanol
E) 1,1-Dimethyl-1-ethanol

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
7
The correct IUPAC substitutive name for 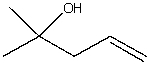 is:
is:
A) 4-Penten-2-methyl-2-ol
B) 4-Methyl-1-penten-2-ol
C) 2-Methyl-4-penten-2-ol
D) 4-Methyl-1-penten-4-ol
E) 4-Hydroxy-4-methyl-1-pentene
 is:
is:A) 4-Penten-2-methyl-2-ol
B) 4-Methyl-1-penten-2-ol
C) 2-Methyl-4-penten-2-ol
D) 4-Methyl-1-penten-4-ol
E) 4-Hydroxy-4-methyl-1-pentene

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these,though often used,is an incorrect common name for (CH3)3COH?
A) tert-Butyl alcohol
B) tert-Butanol
C) 2-Methyl-2-propanol
D) More than one is incorrect.
E) Each is a correct name.
A) tert-Butyl alcohol
B) tert-Butanol
C) 2-Methyl-2-propanol
D) More than one is incorrect.
E) Each is a correct name.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
9
What is the relationship between alcohols I and II? 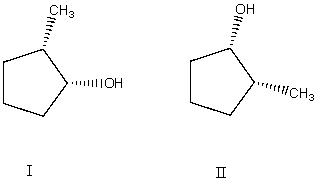 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following can be described as "optically active,primary alcohol"?
A) CH3CH2CH2CH2CH2OH
B) (CH3)2CHCH2CH2OH
C) CH3CH2CH(CH3)CH2OH
D) (CH3)2CHCHOHCH3
E) Two of the above
A) CH3CH2CH2CH2CH2OH
B) (CH3)2CHCH2CH2OH
C) CH3CH2CH(CH3)CH2OH
D) (CH3)2CHCHOHCH3
E) Two of the above

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
11
A correct name for the following Fischer projection formula is: 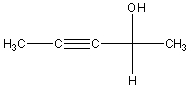
A) (R)-3-Pentyn-2-ol
B) (S)-3-Pentyn-2-ol
C) (R)-2-Pentyn-4-ol
D) (S)-2-Pentyn-4-ol
E) (S)-2-Hydroxy-3-pentyne

A) (R)-3-Pentyn-2-ol
B) (S)-3-Pentyn-2-ol
C) (R)-2-Pentyn-4-ol
D) (S)-2-Pentyn-4-ol
E) (S)-2-Hydroxy-3-pentyne

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct IUPAC name for the following compound? 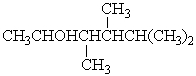
A) 4-isopropyl-3,4-dimethyl-2-butanol
B) 2,3,4-trimethyl-4-pentanol
C) 1,1,2,3-tetramethyl-4-pentanol
D) 3,4,5-trimethyl-2-hexanol
E) 3,4,5,5-tetramethyl-2-pentanol

A) 4-isopropyl-3,4-dimethyl-2-butanol
B) 2,3,4-trimethyl-4-pentanol
C) 1,1,2,3-tetramethyl-4-pentanol
D) 3,4,5-trimethyl-2-hexanol
E) 3,4,5,5-tetramethyl-2-pentanol

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
13
What is the relationship between alcohols I and II? 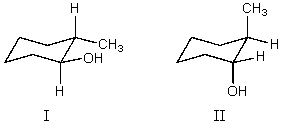 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
14
What is the relationship between alcohols I and II? 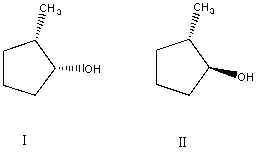 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
15
What is a correct IUPAC name for the following compound? 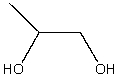
A) 1,2-butanediol
B) isopropanol
C) 1-propanol
D) 1,2-propanediol
E) Ethylene glycol

A) 1,2-butanediol
B) isopropanol
C) 1-propanol
D) 1,2-propanediol
E) Ethylene glycol

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
16
What is the relationship between alcohols I and II? 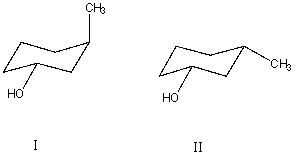 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
17
Which of these,though often used,is an incorrect common name for CH3CHOHCH3?
A) Isopropyl alcohol
B) sec-Propyl alcohol
C) 2-Propanol
D) Isopropanol
E) More than one of these.
A) Isopropyl alcohol
B) sec-Propyl alcohol
C) 2-Propanol
D) Isopropanol
E) More than one of these.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
18
What is the relationship between alcohols I and II? 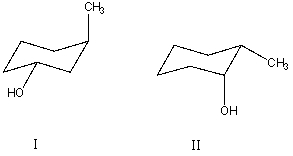 They are:
They are:
A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.
 They are:
They are:A) different conformations of the same compound.
B) constitutional isomers.
C) enantiomers.
D) diastereomers.
E) identical.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
19
What is a correct IUPAC name for the following compound? 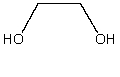
A) 1,2-butanediol
B) isopropanol
C) 1-proanol
D) Propylene glycol
E) 1,2-ethanediol

A) 1,2-butanediol
B) isopropanol
C) 1-proanol
D) Propylene glycol
E) 1,2-ethanediol

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
20
What is the correct IUPAC name for the following compound? 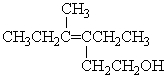
A) 3-methyl-4-ethyl-3-hexen-6-ol
B) 4-ethyl-3-methyl-3,6-hexenol
C) 3-ethyl-4-methyl-3-hexen-1-ol
D) 3-methyl-4-(2-hydroxyethyl)-3-hexene
E) 3-(2-hydroxyethyl)- 3-methyl-3-hexene

A) 3-methyl-4-ethyl-3-hexen-6-ol
B) 4-ethyl-3-methyl-3,6-hexenol
C) 3-ethyl-4-methyl-3-hexen-1-ol
D) 3-methyl-4-(2-hydroxyethyl)-3-hexene
E) 3-(2-hydroxyethyl)- 3-methyl-3-hexene

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight. )
A) CH3CH2CH2CH2CH3
B) CH3CH2CH2CH2OH
C) CH3CH2CH2OCH3
D) CH3CH2CH2Cl
E) CH3CH2OCH2CH3
A) CH3CH2CH2CH2CH3
B) CH3CH2CH2CH2OH
C) CH3CH2CH2OCH3
D) CH3CH2CH2Cl
E) CH3CH2OCH2CH3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
22
The number of primary alcohols corresponding to the formula C5H12O,counting stereoisomers separately,is:
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is NOT true of ethers?
A) Ethers are generally unreactive molecules toward reagents other than strong acids.
B) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight.
C) Ethers generally have much lower water solubilities than alcohols with a corresponding molecular weight.
D) Ethers can generally be cleaved by heating them with strong acids.
E) Ethers form peroxides when allowed to stand in the presence of oxygen.
A) Ethers are generally unreactive molecules toward reagents other than strong acids.
B) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight.
C) Ethers generally have much lower water solubilities than alcohols with a corresponding molecular weight.
D) Ethers can generally be cleaved by heating them with strong acids.
E) Ethers form peroxides when allowed to stand in the presence of oxygen.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
24
The number of optically active pentyl alcohols (C5H11OH),i.e. ,the total number of individual enantiomers,is:
A) 0
B) 2
C) 3
D) 4
E) 6
A) 0
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound would have the lowest boiling point? 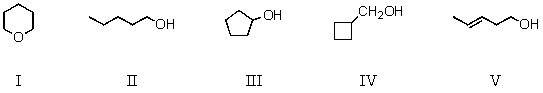
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
26
What is the total number of pentyl alcohols,including stereoisomers?
A) 7
B) 8
C) 9
D) 10
E) 11
A) 7
B) 8
C) 9
D) 10
E) 11

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
27
Which compound would have the highest boiling point?
A) CH3CH2CH2CH3
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH
A) CH3CH2CH2CH3
B) CH3CH2OCH3
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
28
Today,most industrial ethanol is made in the U.S.by the:
A) fermentation of grain.
B) hydrolysis of ethyl bromide.
C) hydration of ethylene.
D) reduction of acetaldehyde.
E) hydration of acetylene.
A) fermentation of grain.
B) hydrolysis of ethyl bromide.
C) hydration of ethylene.
D) reduction of acetaldehyde.
E) hydration of acetylene.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
29
Select the structure of the major product formed from the following reaction. 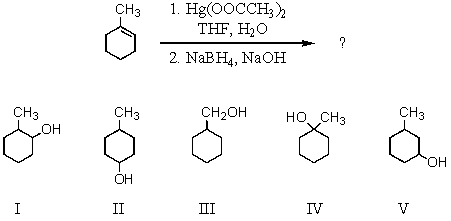
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
30
The major industrial process in use today for the production of methanol is the:
A) hydration of ethyne.
B) distillation of wood.
C) hydrogenation of carbon dioxide.
D) reduction of methanal.
E) catalytic reduction of carbon monoxide.
A) hydration of ethyne.
B) distillation of wood.
C) hydrogenation of carbon dioxide.
D) reduction of methanal.
E) catalytic reduction of carbon monoxide.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound would have the highest boiling point?
A) CH3CH2CH2CH3
B) CH2(OH)CH(OH)CH2OH
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH
A) CH3CH2CH2CH3
B) CH2(OH)CH(OH)CH2OH
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound would have the lowest boiling point?
A) CH3CH2CH2CH3
B) CH3CH2OCH2CH3
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH
A) CH3CH2CH2CH3
B) CH3CH2OCH2CH3
C) CH3CH2CH2OH
D) (CH3)2CHOH
E) HOCH2CH2OH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
33
Which is a correct IUPAC name for CH3CH2OCH2CH2CH2OCH2CH3?
A) 1,4-Dioxane
B) Ethylene glycol diethyl ether
C) 1,3-Diethoxypropane
D) 1,2-Diethoxyethane
E) 1,2-Diethoxymethane
A) 1,4-Dioxane
B) Ethylene glycol diethyl ether
C) 1,3-Diethoxypropane
D) 1,2-Diethoxyethane
E) 1,2-Diethoxymethane

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
34
Which compound would have the lowest solubility in water?
A) Diethyl ether
B) Methyl propyl ether
C) 1-Butanol
D) 2-Butanol
E) Pentane
A) Diethyl ether
B) Methyl propyl ether
C) 1-Butanol
D) 2-Butanol
E) Pentane

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
35
Which compound would have the greatest solubility in water?
A) Diethyl ether
B) Methyl propyl ether
C) 1-Butanol
D) 1,2-Butanediol
E) Pentane
A) Diethyl ether
B) Methyl propyl ether
C) 1-Butanol
D) 1,2-Butanediol
E) Pentane

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
36
The number of secondary alcohols corresponding to the formula C5H12O,counting stereoisomers separately,is:
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
37
The total number of ethers corresponding to the formula C5H12O,counting stereoisomers separately,is:
A) 4
B) 5
C) 6
D) 7
E) 8
A) 4
B) 5
C) 6
D) 7
E) 8

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
38
What is the most accurate name for the molecule represented by the following Fischer projection formula? 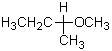
A) sec-Butyl methyl ether
B) Isobutyl methyl ether
C) tert-Butyl methyl ether
D) (R)-2-Methoxybutane
E) (S)-2-Methoxybutane
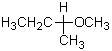
A) sec-Butyl methyl ether
B) Isobutyl methyl ether
C) tert-Butyl methyl ether
D) (R)-2-Methoxybutane
E) (S)-2-Methoxybutane

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
39
The number of tertiary alcohols corresponding to the formula C5H12O,counting stereoisomers separately,is:
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
40
Which product(s)would you expect to obtain from the following sequence of reactions? 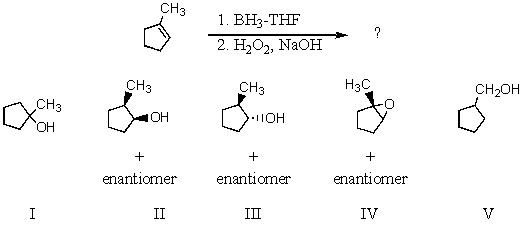
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
41
Which would be the best method for converting 3,3-dimethyl-1-pentene into 3,3-dimethyl-2-pentanol?
A) H3O+,heat
B) BH3:THF;then H2O2,OH-
C) concd.H2SO4;then H2O,heat
D) Hg(OAc)2/THF-H2O;then NaBH4,OH-
E) HBr;then NaOH/H2O
A) H3O+,heat
B) BH3:THF;then H2O2,OH-
C) concd.H2SO4;then H2O,heat
D) Hg(OAc)2/THF-H2O;then NaBH4,OH-
E) HBr;then NaOH/H2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following would be a reasonable synthesis of 2-butanol?
A)
B)
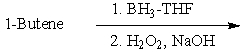
C)
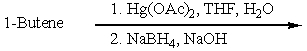
D)
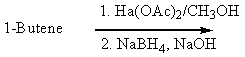
E) None of these
A)
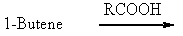
B)
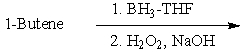
C)
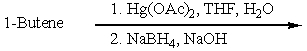
D)
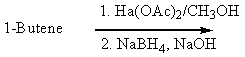
E) None of these

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
43
Select the structure of the major product formed from the following reaction. 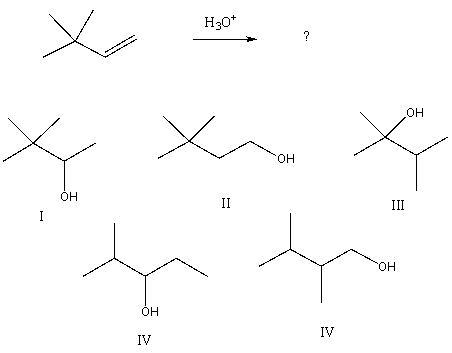
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following would be a reasonable synthesis of CH3CH2CH2CH2OH?
A)
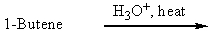
B)
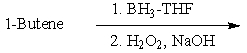
C)
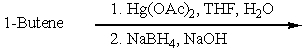
D)
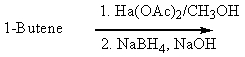
E) None of these
A)
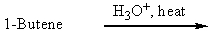
B)
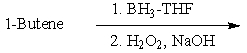
C)
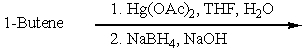
D)
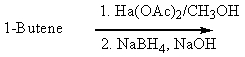
E) None of these

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
45
Select the structure of the major product formed from the following reaction. 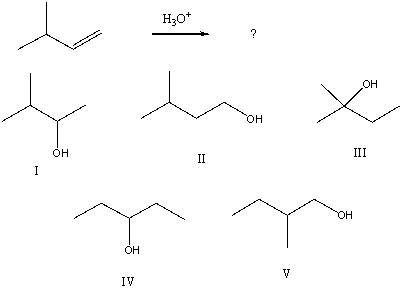
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the alcohols listed below would you expect to react most rapidly with HBr?
A) CH3CH2CH2CH2CH2CH2OH
B) (CH3CH2)2CH2CH2OH
C) (CH3CH2)2CHOHCH3
D) CH3CH2CH2CH2CH2OH
E) (CH3CH2)2C(CH3)OH
A) CH3CH2CH2CH2CH2CH2OH
B) (CH3CH2)2CH2CH2OH
C) (CH3CH2)2CHOHCH3
D) CH3CH2CH2CH2CH2OH
E) (CH3CH2)2C(CH3)OH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
47
Select the potential energy diagram that best represents the following reaction: 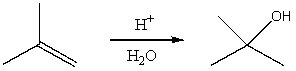
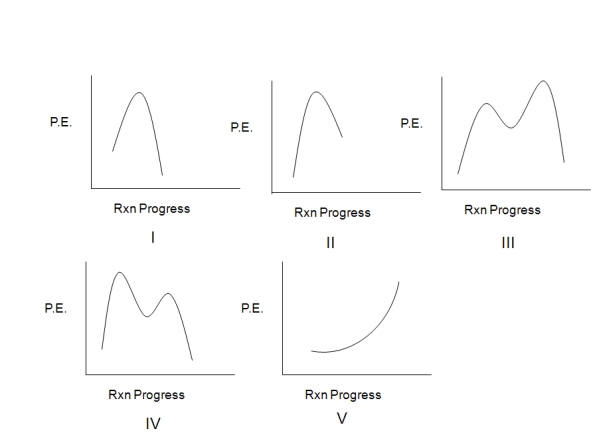
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
48
Oxymercuration-demercuration of 3-methylcyclopentene produces this/these product(s): 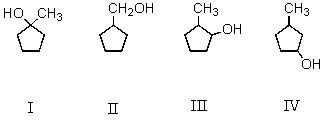
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following could not be used to synthesize 2-bromopentane efficiently?
A) 1-Pentene + HBr
B) 2-Pentene + HBr
C) 2-Pentanol + HBr
D) 2-Pentanol + PBr3
E) All of the above would afford good yields of 2-bromopentane.
A) 1-Pentene + HBr
B) 2-Pentene + HBr
C) 2-Pentanol + HBr
D) 2-Pentanol + PBr3
E) All of the above would afford good yields of 2-bromopentane.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
50
Which statement is true concerning the formation of alcohols by the hydroboration-oxidation sequence?
A) Overall,the process results in syn addition and anti-Markovnikov orientation.
B) Overall,the process results in anti addition and anti-Markovnikov orientation.
C) Overall,the process results in syn addition and Markovnikov orientation.
D) Overall,the process results in anti addition and Markovnikov orientation.
E) The stereochemistry and orientation are unpredictable.
A) Overall,the process results in syn addition and anti-Markovnikov orientation.
B) Overall,the process results in anti addition and anti-Markovnikov orientation.
C) Overall,the process results in syn addition and Markovnikov orientation.
D) Overall,the process results in anti addition and Markovnikov orientation.
E) The stereochemistry and orientation are unpredictable.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
51
What is the electrophilic species involved in the initial step of the reaction below? 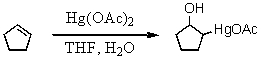
A) "+OH"
B) "+HgOAc"
C) "H3O+"
D) "THF"
E) "the THF/H2O complex"
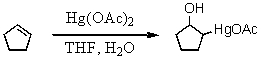
A) "+OH"
B) "+HgOAc"
C) "H3O+"
D) "THF"
E) "the THF/H2O complex"

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
52
The hydroboration-oxidation procedure can be successfully employed for synthesis of deuterated derivatives,by using BD3 instead of BH3.What product would you expect from the following reaction? 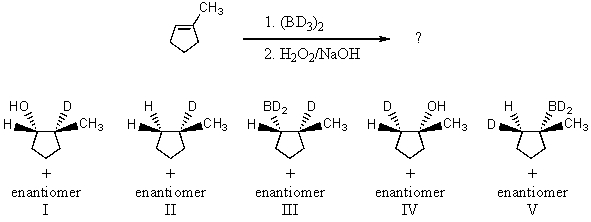
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
53
Assuming an overall exothermic process,select the potential energy diagram that best represents the following reaction: 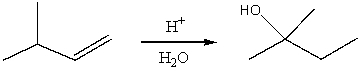
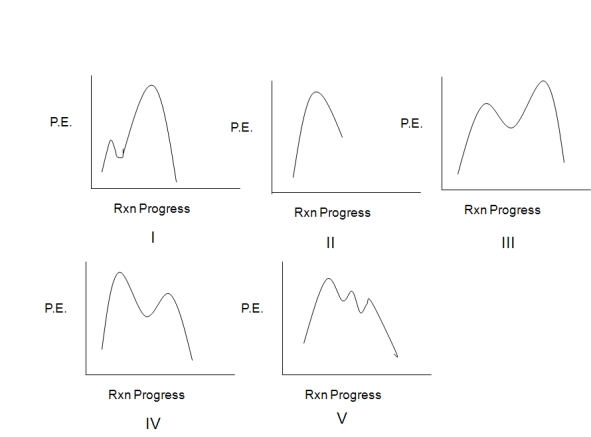
A) I
B) II
C) III
D) IV
E) V


A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
54
Which would be the major product of the reaction shown? 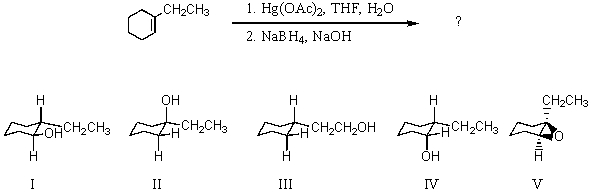
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
55
Which alcohol would undergo acid-catalyzed dehydration most rapidly?
A) 3,3-dimethyl-1-butanol
B) 2,2-dimethyl-1-butanol
C) 3,3-dimethyl-2-butanol
D) 2-methyl-2-butanol
E) All would undergo dehydration equally rapidly.
A) 3,3-dimethyl-1-butanol
B) 2,2-dimethyl-1-butanol
C) 3,3-dimethyl-2-butanol
D) 2-methyl-2-butanol
E) All would undergo dehydration equally rapidly.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
56
Anti-Markovnikov hydration of the carbon-carbon double bond occurs when an alkene reacts with:
A) BH3:THF;then H2O2/OH-
B) BH3:THF;then CH3COOH
C) Hg(OAc)2,THF,H2O;then NaBH4,OH-
D) Hg(OAc)2,THF,CH3OH;then NaBH4,OH-
E) Hg(OAc)2,THF,H2O;then BH3:THF
A) BH3:THF;then H2O2/OH-
B) BH3:THF;then CH3COOH
C) Hg(OAc)2,THF,H2O;then NaBH4,OH-
D) Hg(OAc)2,THF,CH3OH;then NaBH4,OH-
E) Hg(OAc)2,THF,H2O;then BH3:THF

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
57
What is the nucleophilic species involved in the initial step of the reaction below? 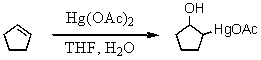
A) "-OH"
B) "Hg(OAc)2"
C) "H2O"
D) "cyclopentene"
E) "the THF/H2O complex"
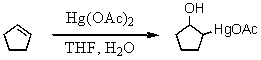
A) "-OH"
B) "Hg(OAc)2"
C) "H2O"
D) "cyclopentene"
E) "the THF/H2O complex"

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
58
Which would be the best way to carry out the following synthesis? 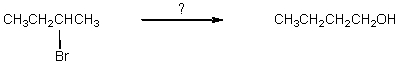
A) (1)HA,heat; (2)H3O+,H2O,heat
B) (1)(CH3)3COK / (CH3)3COH; (2)BH3:THF,then H2O2,OH-
C) (1)(CH3)3COK / (CH3)3COH; (2)H3O+,then H2O,heat
D) (1)KOH,C2H5OH; (2)BH3:THF,then H2O2,OH-
E) (1)KOH,C2H5OH; (2)HA,heat; (3)H3O+,H2O,heat
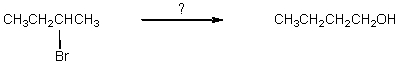
A) (1)HA,heat; (2)H3O+,H2O,heat
B) (1)(CH3)3COK / (CH3)3COH; (2)BH3:THF,then H2O2,OH-
C) (1)(CH3)3COK / (CH3)3COH; (2)H3O+,then H2O,heat
D) (1)KOH,C2H5OH; (2)BH3:THF,then H2O2,OH-
E) (1)KOH,C2H5OH; (2)HA,heat; (3)H3O+,H2O,heat

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
59
The oxymercuration-demercuration procedure can be successfully employed for synthesis of deuterated derivatives,by using NaBD4 instead of NaBH4 in the second step.What product would you expect from the following reaction? 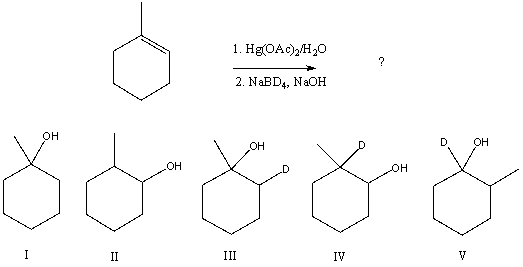
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these alkyl halide syntheses is predicted to occur at the greatest rate?
A) CH3CH2CH2CH2OH + HI
B) (CH3)2CHCH2OH + HBr
C) CH3CHOHCH2CH3 + HCl
D) CH3CHOHCH2CH3 + HBr
E) (CH3)3COH + HI
A) CH3CH2CH2CH2OH + HI
B) (CH3)2CHCH2OH + HBr
C) CH3CHOHCH2CH3 + HCl
D) CH3CHOHCH2CH3 + HBr
E) (CH3)3COH + HI

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following could be used to synthesize 2-chlorobutane?
A) CH3CH2CH CH2 + Cl2 (aq)
B) CH3CH2C CH3 + HCl
C) CH3CH2C CH + HCl
D) CH3CH2C CH + Cl2
E) None of the above
A) CH3CH2CH CH2 + Cl2 (aq)
B) CH3CH2C CH3 + HCl
C) CH3CH2C CH + HCl
D) CH3CH2C CH + Cl2
E) None of the above

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
62
cis-3-Methylcyclopentanol is treated with CH3SO2Cl in the presence of a base.The product of the reaction then is allowed to react with KI in methanol.What is the final product?
A) trans-1-Iodo-3-methylcyclopentane
B) cis-1-Iodo-3-methylcyclopentane
C) 1-Methylcyclopentene
D) 2-Methylcyclopentene
E) 3-Methylcyclopentene
A) trans-1-Iodo-3-methylcyclopentane
B) cis-1-Iodo-3-methylcyclopentane
C) 1-Methylcyclopentene
D) 2-Methylcyclopentene
E) 3-Methylcyclopentene

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
63
trans-3-Methylcyclopentanol is treated with CH3SO2Cl in the presence of base.The product of this reaction is then heated with KI in methanol.What is the final product?
A) trans-1-Iodo-3-methylcyclopentane
B) cis-1-Iodo-3-methylcyclopentane
C) 1-Methylcyclopentene
D) 2-Methylcyclopentene
E) 3-Methylcyclopentene
A) trans-1-Iodo-3-methylcyclopentane
B) cis-1-Iodo-3-methylcyclopentane
C) 1-Methylcyclopentene
D) 2-Methylcyclopentene
E) 3-Methylcyclopentene

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the alcohols listed below would you expect to react most rapidly with PBr3?
A) CH3CH2CH2CH2CH2CH2OH
B) (CH3CH2)2CH(OH)CH2CH3
C) (CH3CH2)2CHOHCH3
D) (CH3CH2)3COH
E) (CH3CH2)2C(CH3)OH
A) CH3CH2CH2CH2CH2CH2OH
B) (CH3CH2)2CH(OH)CH2CH3
C) (CH3CH2)2CHOHCH3
D) (CH3CH2)3COH
E) (CH3CH2)2C(CH3)OH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
65
What would be the major product of the following reaction sequence? 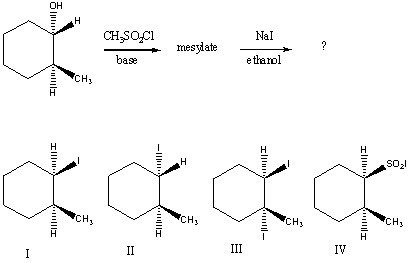
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following could be used to synthesize 1-bromobutane efficiently?
A) CH3CH2CH=CH2 + HBr
B) CH3CH2CH2CH2OH + PBr3
C) CH3CH2CH2(OH)CH3 + HBr
D) CH3CH2CH2CH2OH + Br2
E) None of these
A) CH3CH2CH=CH2 + HBr
B) CH3CH2CH2CH2OH + PBr3
C) CH3CH2CH2(OH)CH3 + HBr
D) CH3CH2CH2CH2OH + Br2
E) None of these

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
67
Which reagent(s)would transform propyl alcohol into propyl bromide?
A) Concd.HBr and heat
B) PBr3
C) NaBr/H2O and heat
D) More than one of these
E) All of these
A) Concd.HBr and heat
B) PBr3
C) NaBr/H2O and heat
D) More than one of these
E) All of these

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
68
The conversion of 3-methyl-1-octanol to 1-chloro-3-methyloctane is best achieved through use of which of these reagents?
A) "Concd.HCl"
B) "SO2Cl2"
C) "NaCl,H2SO4"
D) "PCl3"
E) " POCl3"
A) "Concd.HCl"
B) "SO2Cl2"
C) "NaCl,H2SO4"
D) "PCl3"
E) " POCl3"

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following could be used to synthesize 2-iodobutane?
A) CH3CH2CH CH2 + I2 (aq)
B) CH3CH2C CH3 + HI
C) CH3CH2C CH + HI
D) CH3CH2C CH + I2
E) None of the above
A) CH3CH2CH CH2 + I2 (aq)
B) CH3CH2C CH3 + HI
C) CH3CH2C CH + HI
D) CH3CH2C CH + I2
E) None of the above

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following could be used to synthesize 1-bromopentane?
A) CH3CH2CH2CH=CH2 + HBr
B) CH3CH2CH2CH2CH2OH + PBr3
C) CH3CH2CH2CH2CH2OH + NaBr
D) CH3CH2CH2CH2CH2OH + Br2
E) CH3CH2CH2CH=CH2 + Br2
A) CH3CH2CH2CH=CH2 + HBr
B) CH3CH2CH2CH2CH2OH + PBr3
C) CH3CH2CH2CH2CH2OH + NaBr
D) CH3CH2CH2CH2CH2OH + Br2
E) CH3CH2CH2CH=CH2 + Br2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
71
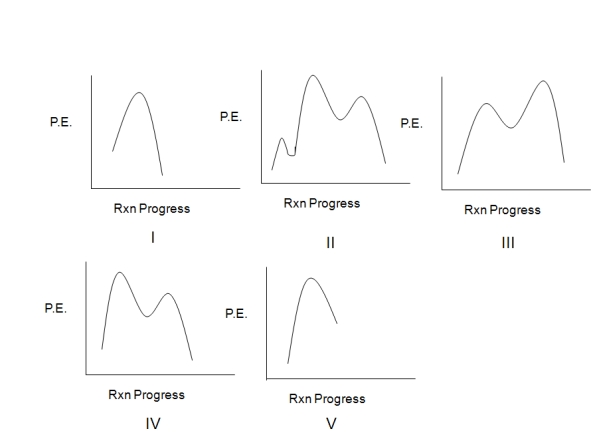
Assuming an overall exothermic process,select the potential energy diagram that best represents the following reaction: 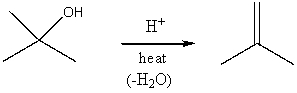
A) I
B) II
C) III
D) IV
E) V
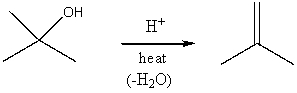

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
72
What would be the major product of the following reaction sequence? 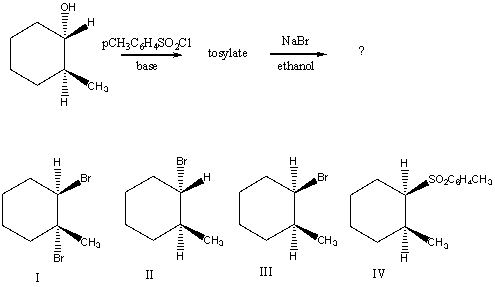
A) I
B) II
C) III
D) IV
E) An equimolar mixture of II and III

A) I
B) II
C) III
D) IV
E) An equimolar mixture of II and III

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
73
What would be the major product of the following reaction sequence? 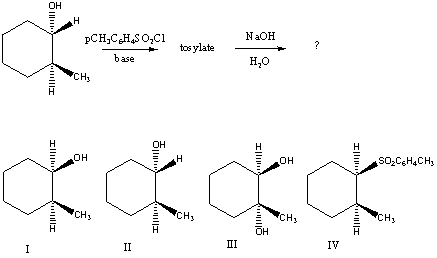
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
74
The conversion of 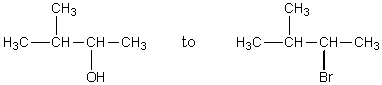 is best achieved through use of which of these reagents in a low temperature reaction?
is best achieved through use of which of these reagents in a low temperature reaction?
A) Concd.HBr
B) Br2
C) NaBr,H2SO4
D) PBr3
E) HBr,peroxide
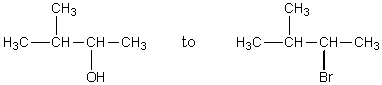 is best achieved through use of which of these reagents in a low temperature reaction?
is best achieved through use of which of these reagents in a low temperature reaction?A) Concd.HBr
B) Br2
C) NaBr,H2SO4
D) PBr3
E) HBr,peroxide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
75
Which compound is a tosylate? 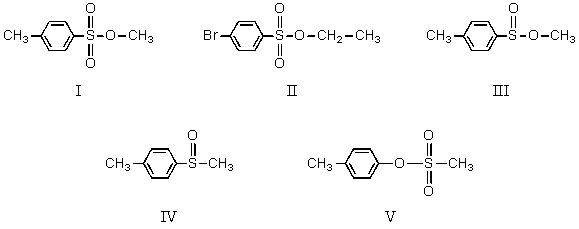
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
76
The following reaction,  is probably:
is probably:
A) an SN1-type reaction involving the protonated alcohol as the substrate.
B) an SN2-type reaction involving the protonated alcohol as the substrate.
C) an E1-type reaction involving the protonated alcohol as the substrate.
D) an E2-type reaction involving the protonated alcohol as the substrate.
E) an epoxidation reaction.
 is probably:
is probably:A) an SN1-type reaction involving the protonated alcohol as the substrate.
B) an SN2-type reaction involving the protonated alcohol as the substrate.
C) an E1-type reaction involving the protonated alcohol as the substrate.
D) an E2-type reaction involving the protonated alcohol as the substrate.
E) an epoxidation reaction.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
77
The major product of the following reaction would be: 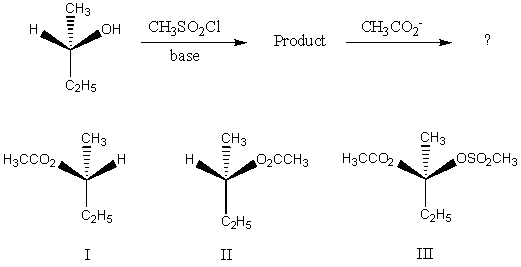
A) I
B) II
C) III
D) Equal amounts of I and II
E) None of these

A) I
B) II
C) III
D) Equal amounts of I and II
E) None of these

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
78
What would be the major product of the following reaction sequence? 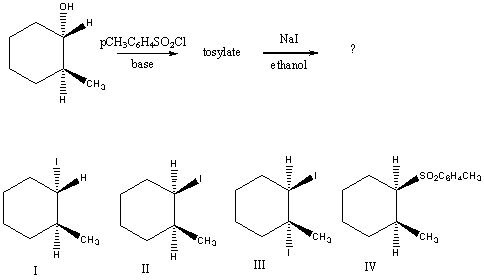
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following could be used to synthesize 2-bromobutane?
A) CH3CH2CH CH2 + Br2 (aq)
B) CH3CH2C CH3 + HBr
C) CH3CH2C CH + HBr
D) CH3CH2C CH + Br2
E) More than one of the above
A) CH3CH2CH CH2 + Br2 (aq)
B) CH3CH2C CH3 + HBr
C) CH3CH2C CH + HBr
D) CH3CH2C CH + Br2
E) More than one of the above

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
80
What would be the major product of the following reaction sequence? 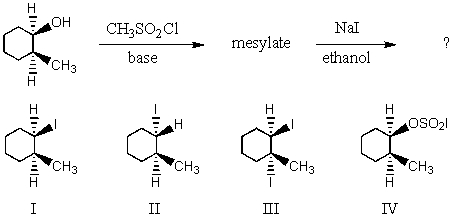
A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

A) I
B) II
C) III
D) IV
E) An equimolar mixture of I and II

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck


