Deck 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/165
Play
Full screen (f)
Deck 16: Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
1
What is the correct structure for 3-methyl-5-(2,5-dinitrophenyl)pentanal? 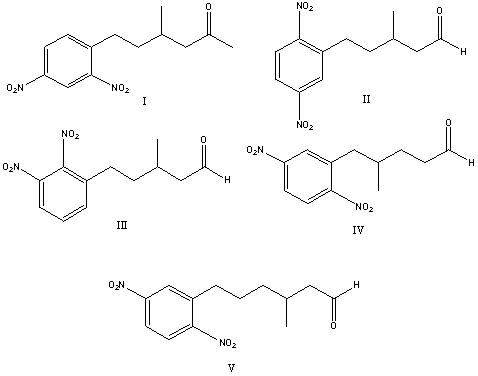
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
II
2
What is the correct IUPAC name for the following compound? 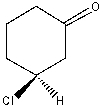
A) (S)-3-chloro-1-hexanone
B) (S)-1-chloro-3-hexanone
C) (R)-3-chlorocyclohexanone
D) (S)-3-chlorocyclohexanone
E) (S)-1-chloro-3-cyclohexanone

A) (S)-3-chloro-1-hexanone
B) (S)-1-chloro-3-hexanone
C) (R)-3-chlorocyclohexanone
D) (S)-3-chlorocyclohexanone
E) (S)-1-chloro-3-cyclohexanone
(S)-3-chlorocyclohexanone
3
What is the correct structure for 5,5-dimethyl-2-heptanone? 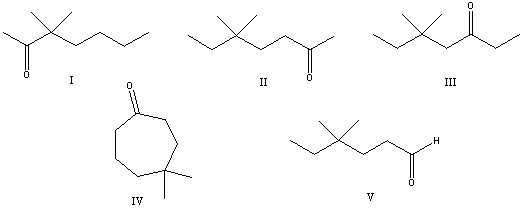
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
II
4
What is the correct IUPAC name for the following compound? 
A) 5,5-Dimethyl-2-heptanone
B) 5-Ethyl-5,5-dimethyl-Methyl-2-octanone
C) 5-Ethyl-5-methyl- 2-hexanone
D) 5,5-Dimethyl-2-octanone
E) 3,3-Dimethyl-6-heptanone

A) 5,5-Dimethyl-2-heptanone
B) 5-Ethyl-5,5-dimethyl-Methyl-2-octanone
C) 5-Ethyl-5-methyl- 2-hexanone
D) 5,5-Dimethyl-2-octanone
E) 3,3-Dimethyl-6-heptanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
5
What is the correct structure for 4-chloro-3-methylbenzaldehyde? 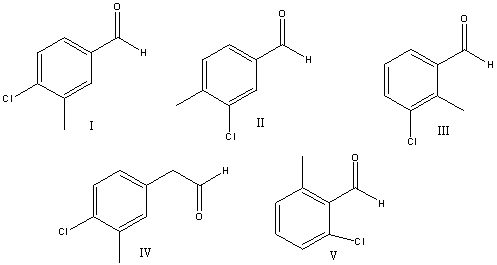
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
6
What is the correct IUPAC name for the following compound? 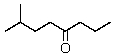
A) 2-Methyl-5-heptanone
B) 7-Methyl-4-octanone
C) 6-Isopropyl-4-octanone
D) Isobutyl propyl ketone
E) 1,1-Dimethyl-4-heptanone

A) 2-Methyl-5-heptanone
B) 7-Methyl-4-octanone
C) 6-Isopropyl-4-octanone
D) Isobutyl propyl ketone
E) 1,1-Dimethyl-4-heptanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct IUPAC name for the following compound? 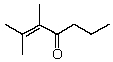
A) 1,1,2-Trimethyl-1,3-hexenone
B) 1,2-Dimethyl-1,3-hexenone
C) 2,3-Dimethyl-1,3-heptenone
D) 2,3-Dimethyl-2-hepten-4-one
E) 5,6-Dimethyl-5-hepten-4-one

A) 1,1,2-Trimethyl-1,3-hexenone
B) 1,2-Dimethyl-1,3-hexenone
C) 2,3-Dimethyl-1,3-heptenone
D) 2,3-Dimethyl-2-hepten-4-one
E) 5,6-Dimethyl-5-hepten-4-one

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
8
What is the correct structure for 7-bromo-1-octyn-4-one? 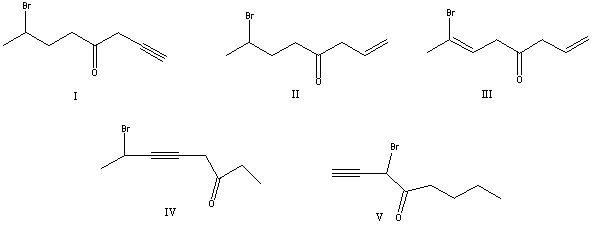
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
9
What is the correct structure for 3-methyl-5-(4-chlorophenyl)hexanal? 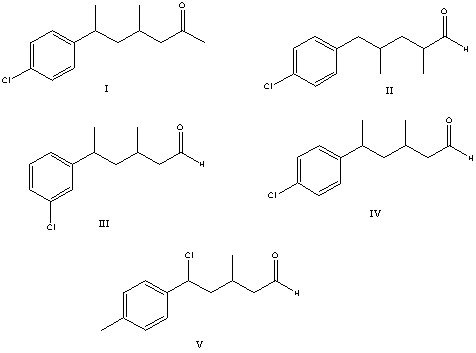
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
10
A correct name for the following compound would be which of those below? 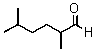
A) 2,5-Dimethyl-6-hexanal
B) 2,5-Dimethylhexanal
C) 2-Aldehydoisohexane
D) 3,5-Dimethylheptanone
E) 1-Hydro-2,5-dimethyl-1-hexanone

A) 2,5-Dimethyl-6-hexanal
B) 2,5-Dimethylhexanal
C) 2-Aldehydoisohexane
D) 3,5-Dimethylheptanone
E) 1-Hydro-2,5-dimethyl-1-hexanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the proper name for the structure shown? 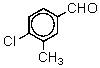
A) 2-Chloro-5-aldehydotoluene
B) 6-Chloro-3-aldehydotoluene
C) 2-Methyl-4-aldehydochlorobenzene
D) 4-Chloro-3-methylbenzaldehyde
E) 4-Methyl-5-chloro-2-benzaldehyde

A) 2-Chloro-5-aldehydotoluene
B) 6-Chloro-3-aldehydotoluene
C) 2-Methyl-4-aldehydochlorobenzene
D) 4-Chloro-3-methylbenzaldehyde
E) 4-Methyl-5-chloro-2-benzaldehyde

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct structure for 2,3-dimethyl-2-hepten-4-one? 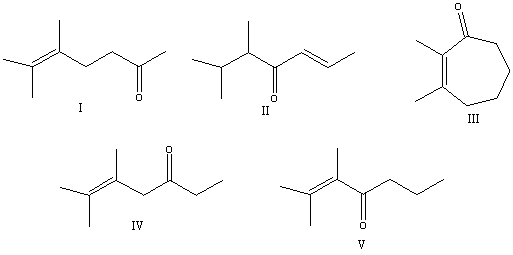
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct IUPAC name for the following compound? 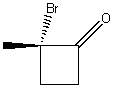
A) 2-Methyl-2-bromobutanone
B) (S)-2-Bromo-2-methylcyclobutanone
C) (R)-2-Bromo-2-methylcyclobutanone
D) (S)-1-Bromo-1-methyl-2-cyclobutanone
E) (R)-1-Bromo-1-methyl-2-cyclobutanone

A) 2-Methyl-2-bromobutanone
B) (S)-2-Bromo-2-methylcyclobutanone
C) (R)-2-Bromo-2-methylcyclobutanone
D) (S)-1-Bromo-1-methyl-2-cyclobutanone
E) (R)-1-Bromo-1-methyl-2-cyclobutanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct IUPAC name for the following compound? 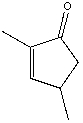
A) 2,4-dimethyl-2-pentenone
B) 2,5-dimethylcyclopenten-3-one
C) 2,4-dimethylcyclopent-2-enone
D) 3,5-dimethylcyclopent-2-enone
E) 2-methyl-5-methylcyclopent-2-enone

A) 2,4-dimethyl-2-pentenone
B) 2,5-dimethylcyclopenten-3-one
C) 2,4-dimethylcyclopent-2-enone
D) 3,5-dimethylcyclopent-2-enone
E) 2-methyl-5-methylcyclopent-2-enone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
15
What is the correct structure for 3-phenylpropanal? 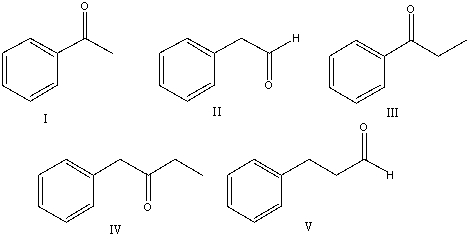
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
16
What is the correct IUPAC name for the following compound? 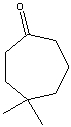
A) 5,5-Dimethyl-2-heptanone
B) 5-Ethyl-5,5-dimethyl-Methyl-2-octanone
C) 5,5-Dimethylcycloheptanone
D) 4,4-Dimethylcycloheptanone
E) 3,3-Dimethylcycloheptanone

A) 5,5-Dimethyl-2-heptanone
B) 5-Ethyl-5,5-dimethyl-Methyl-2-octanone
C) 5,5-Dimethylcycloheptanone
D) 4,4-Dimethylcycloheptanone
E) 3,3-Dimethylcycloheptanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
17
What is the correct structure for 5-hydroxy-2-phenyl-3-hexanone? 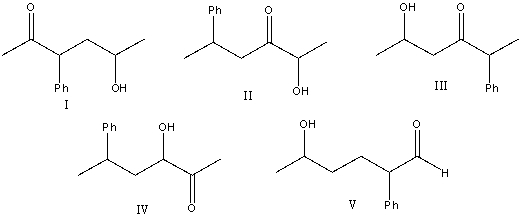
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
18
What is the correct structure for 7-methyl-4-octanone? 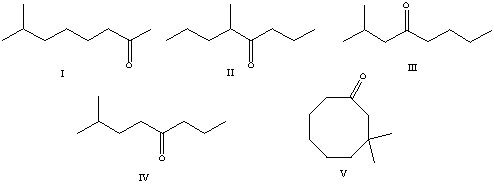
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
19
What is the correct structure for 2,5-dimethylhexanal? 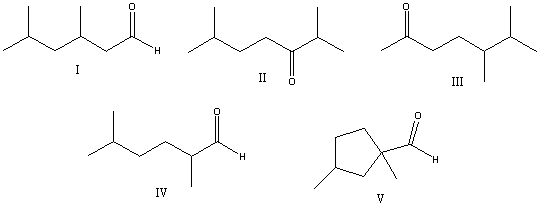
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
20
A correct name for 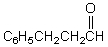 is?
is?
A) 3-Benzylpropanone
B) 3-Phenylpropanal
C) 3-Benzylpropanal
D) Nonanone
E) Nonanal
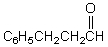 is?
is?A) 3-Benzylpropanone
B) 3-Phenylpropanal
C) 3-Benzylpropanal
D) Nonanone
E) Nonanal

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
21
What is the final product,Z,of the following synthesis? 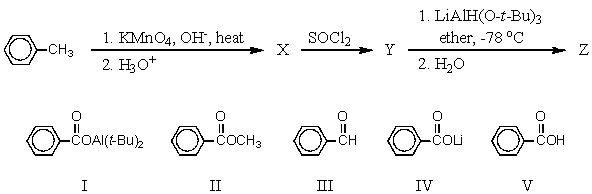
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
22
What is the correct structure for (S)-7-bromo-1-octyn-4-one? 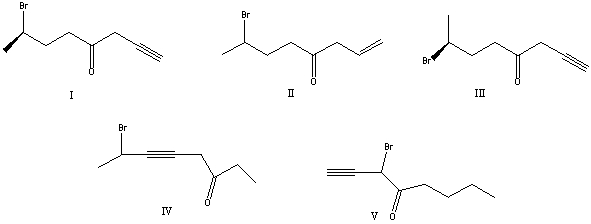
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
23
Which is the IUPAC name for the structure shown below? 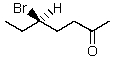
A) (R)-5-Bromo-2-heptanal
B) (S)-5-Bromo-2-heptanal
C) (R)-5-Bromo-2-heptanone
D) (S)-5-Bromo-2-heptanone
E) (R)-3-Bromopentyl methyl ketone

A) (R)-5-Bromo-2-heptanal
B) (S)-5-Bromo-2-heptanal
C) (R)-5-Bromo-2-heptanone
D) (S)-5-Bromo-2-heptanone
E) (R)-3-Bromopentyl methyl ketone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
24
What is the correct structure for (R)-7-bromo-1-octyn-4-one? 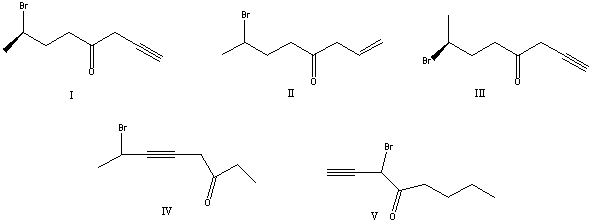
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
25
Select the structure of the major product in the following reaction. 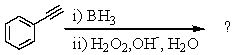
A) Ethylbenzene
B) 1-Phenylethanol
C) Acetophenone
D) 2-Phenylethanal
E) Vinylbenzene
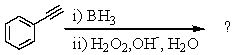
A) Ethylbenzene
B) 1-Phenylethanol
C) Acetophenone
D) 2-Phenylethanal
E) Vinylbenzene

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight. )
A) Pentane
B) 1-Butanol
C) Butanal
D) 1-Fluorobutane
E) Diethylether
A) Pentane
B) 1-Butanol
C) Butanal
D) 1-Fluorobutane
E) Diethylether

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct structure for (R)-5-bromo-2-heptanone? 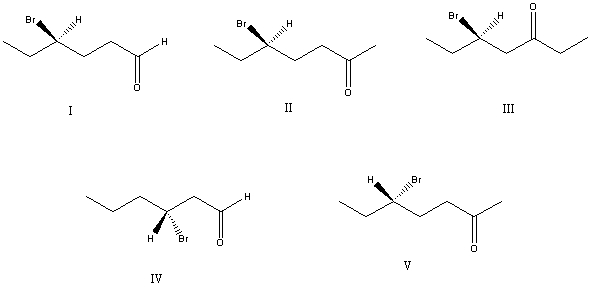
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
28
What is the correct structure for (S)-5-bromo-2-heptanone? 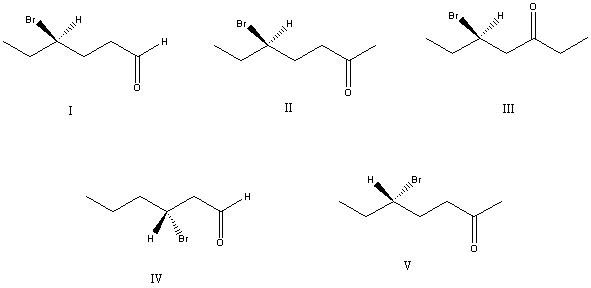
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
29
Which is the IUPAC name for the structure shown below? 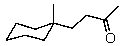
A) 5-Cyclohexyl-2-hexanal
B) 5-Cyclohexyl-2-hexanone
C) 5-Cyclohexyl-5-methyl-2-pentanone
D) 5-(1-Methylcyclohexyl)-2-pentanone
E) 4-(1-Methylcyclohexyl)-2-butanone

A) 5-Cyclohexyl-2-hexanal
B) 5-Cyclohexyl-2-hexanone
C) 5-Cyclohexyl-5-methyl-2-pentanone
D) 5-(1-Methylcyclohexyl)-2-pentanone
E) 4-(1-Methylcyclohexyl)-2-butanone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
30
Which is the proper name for the structure shown below? 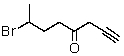
A) 7-Bromo-1,4-octynal
B) 7-Bromo-1,4-octynone
C) 2-Bromo-5,7-octynal
D) 7-Bromo-7-methyl-1-heptyne-3-ketone
E) 7-Bromo-1-octyn-4-one

A) 7-Bromo-1,4-octynal
B) 7-Bromo-1,4-octynone
C) 2-Bromo-5,7-octynal
D) 7-Bromo-7-methyl-1-heptyne-3-ketone
E) 7-Bromo-1-octyn-4-one

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
31
What is the correct structure for (S)-4-cyclohexyl-3-methyl-2-butanone? 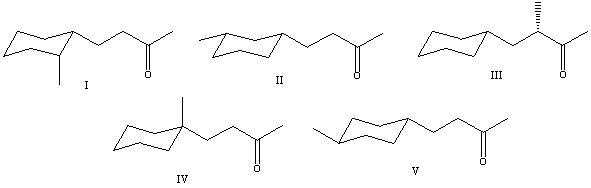
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
32
Select the structure of the major product in the following reaction. 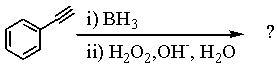
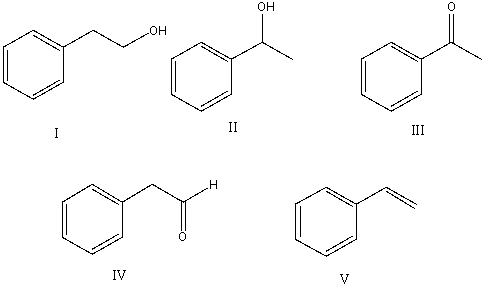
A) I
B) II
C) III
D) IV
E) V
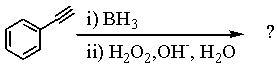

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following procedures would not yield 3-pentanone as a major product?
A)
B)
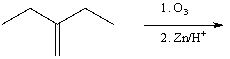
C)
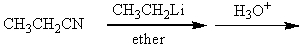
D)
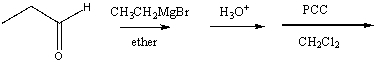
E)
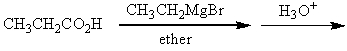
A)
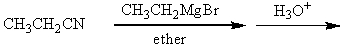
B)

C)
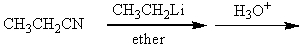
D)
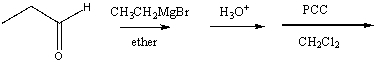
E)
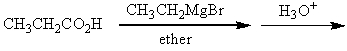

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
34
Which is the proper name for the structure shown? 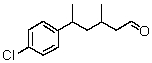
A) 3,5-Dimethyl-5-(4-chlorobenzyl)-1-pentanal
B) 3-Methyl-5-(4-chlorophenyl)hexanol
C) 3,5-Dimethyl-5-(4-chlorophenyl)-1-pentanaldehyde
D) 3-Methyl-5-(4-chlorophenyl)-hexanaldehyde
E) 3-Methyl-5-(4-chlorophenyl)hexanal

A) 3,5-Dimethyl-5-(4-chlorobenzyl)-1-pentanal
B) 3-Methyl-5-(4-chlorophenyl)hexanol
C) 3,5-Dimethyl-5-(4-chlorophenyl)-1-pentanaldehyde
D) 3-Methyl-5-(4-chlorophenyl)-hexanaldehyde
E) 3-Methyl-5-(4-chlorophenyl)hexanal

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name for 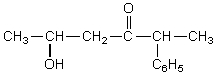 ?
?
A) 4-Oxo-5-phenyl-2-hexanol
B) 5-Hydroxy-2-phenyl-3-hexanone
C) 2-Hydroxy-5-phenyl-4-hexanone
D) 2-Hydroxypropyl-1-phenylethyl ketone
E) 5-Hydroxy-3-keto-2-phenylhexane
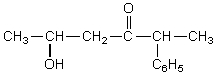 ?
?A) 4-Oxo-5-phenyl-2-hexanol
B) 5-Hydroxy-2-phenyl-3-hexanone
C) 2-Hydroxy-5-phenyl-4-hexanone
D) 2-Hydroxypropyl-1-phenylethyl ketone
E) 5-Hydroxy-3-keto-2-phenylhexane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
36
What is the correct structure for (S)-4-cyclohexyl-3-methyl-2-butanone? 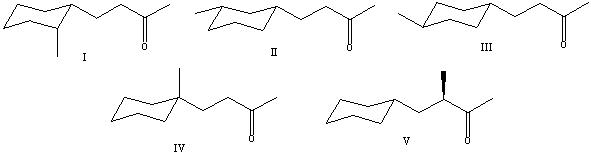
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the IUPAC name for the structure shown below? 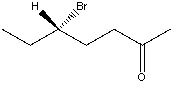
A) (R)-5-Bromo-2-heptanal
B) (S)-5-Bromo-2-heptanal
C) (R)-5-Bromo-2-heptanone
D) (S)-5-Bromo-2-heptanone
E) (R)-3-Bromopentyl methyl ketone

A) (R)-5-Bromo-2-heptanal
B) (S)-5-Bromo-2-heptanal
C) (R)-5-Bromo-2-heptanone
D) (S)-5-Bromo-2-heptanone
E) (R)-3-Bromopentyl methyl ketone

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the reagent(s)that would bring about the following reaction: CH3CH2CH2COCl -- CH3CH2CH2CHO
A) H2/Ni
B) Li/liq.NH3
C) LiAlH[OC(CH3)3]3,ether
D) NaBH4,CH3OH
E) LiAlH4,ether
A) H2/Ni
B) Li/liq.NH3
C) LiAlH[OC(CH3)3]3,ether
D) NaBH4,CH3OH
E) LiAlH4,ether

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
39
LiAlH4 (LAH)cannot be used to convert carboxylic acids to the corresponding aldehydes because:
A) LAH is not sufficiently reactive.
B) RCOOH is converted into RCOOLi.
C) RCOOH is reduced to RCH2OH.
D) RCOOH is reduced to RCH3.
E) RCOOH is converted into R2C=O.
A) LAH is not sufficiently reactive.
B) RCOOH is converted into RCOOLi.
C) RCOOH is reduced to RCH2OH.
D) RCOOH is reduced to RCH3.
E) RCOOH is converted into R2C=O.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
40
What is the correct structure for 4-(1-methylcyclohexyl)-2-butanone? 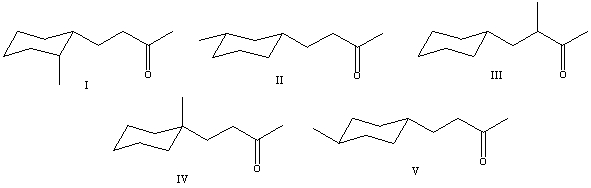
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
41
Select the structure of the major product in the following reaction. 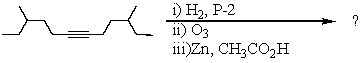
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol
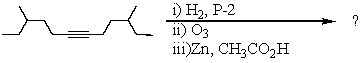
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
42
Select the structure of the major product in the following reaction. 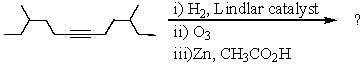
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol
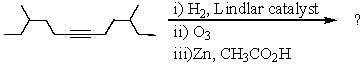
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
43
Select the structure of the major product in the following reaction. 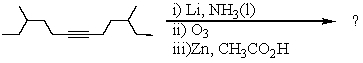
A) 3-Methylhexanal
B) 4-Methyl-1-hexanol
C) 4-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol
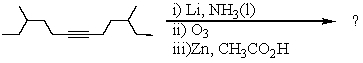
A) 3-Methylhexanal
B) 4-Methyl-1-hexanol
C) 4-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
44
A good synthesis of 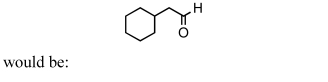
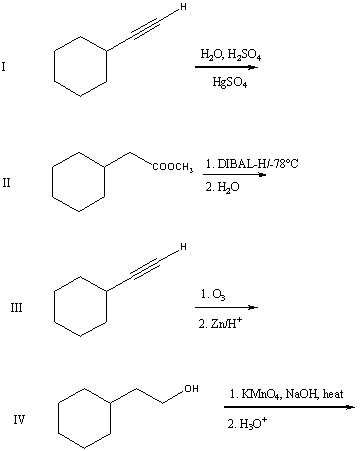
A) I
B) II
C) III
D) IV
E) All of these are equally useful.


A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
45
A good synthesis of 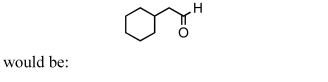
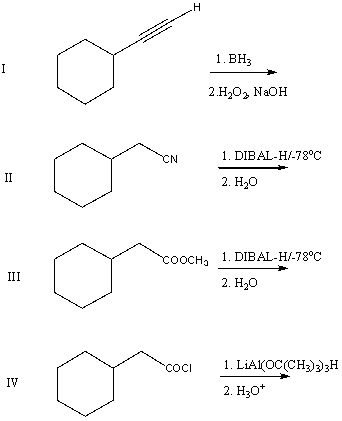
A) I
B) II
C) III
D) IV
E) All of these are equally useful.


A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
46
Select the structure of the major product in the following reaction. 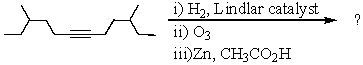
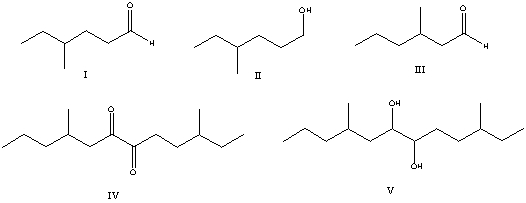
A) I
B) II
C) III
D) IV
E) V
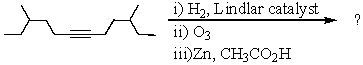

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
47
Select the structure of the major product in the following reaction. 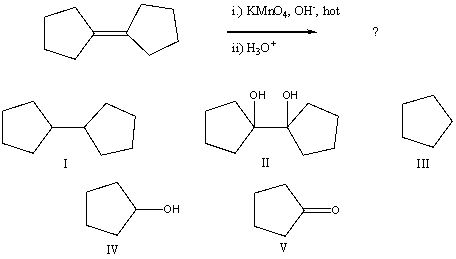
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
48
A good synthesis of 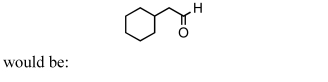
A) I
B) II
C) III
D) IV
E) All of these are equally useful.


A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
49
A good synthesis of 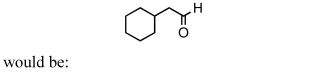
A) I
B) II
C) III
D) IV
E) All of these are equally useful.


A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
50
Select the structure of the major product in the following reaction. 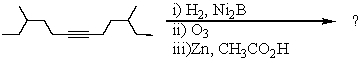
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol
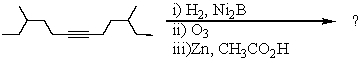
A) 4-Methylhexanal
B) 4-Methyl-1-hexanol
C) 3-Methylhexanal
D) 4,10-Dimethyldodecane-6,7-dione
E) 4,10-Dimethyldodecane-6,7-diol

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
51
Select the structure of the major product in the following reaction. 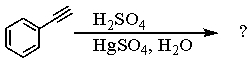
A) I
B) II
C) III
D) IV
E) V
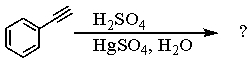

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
52
Which synthesis or syntheses would yield propanal?
A)
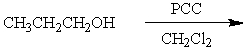
B)
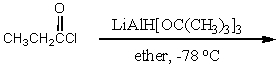
C)
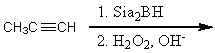
D) All of these
E) None of these
A)
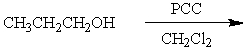
B)
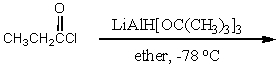
C)
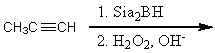
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following would yield 3-pentanone as the major product?
A)
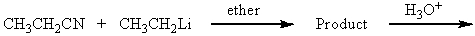
B)
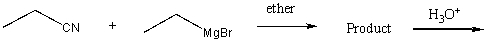
C)
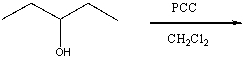
D) Two of these
E) All of these
A)
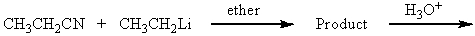
B)
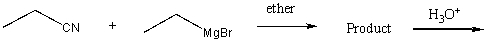
C)

D) Two of these
E) All of these

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
54
A good synthesis of 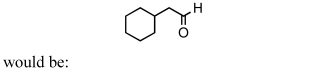
A) I
B) II
C) III
D) IV
E) All of these are equally useful.


A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
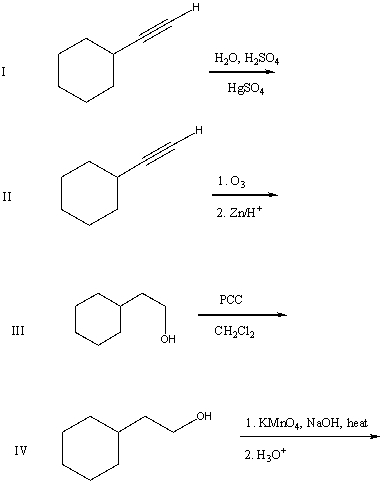
55
What would be the product of the following reaction sequence? 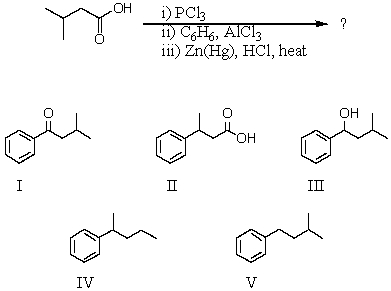
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
56
A good synthesis of 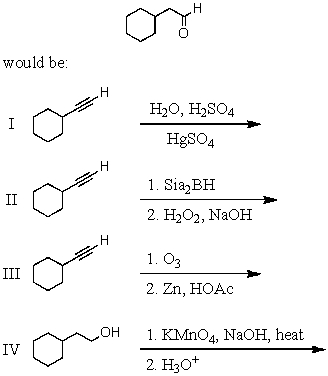
A) I
B) II
C) III
D) IV
E) All of these are equally useful.

A) I
B) II
C) III
D) IV
E) All of these are equally useful.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
57
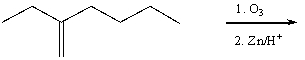
Which of the following is a synthesis of 3-heptanone?
A)
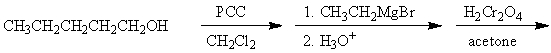
B)
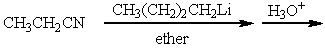
C)
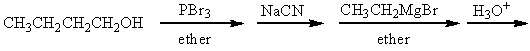
D)
E) All of the above are syntheses of 3-heptanone.
A)
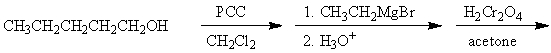
B)
C)
D)

E) All of the above are syntheses of 3-heptanone.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
58
Select the structure of the major product in the following reaction. 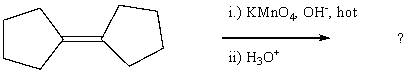
A) cyclopentanol
B) 1-cyclopentylcyclopentane
C) cyclopentanone
D) cyclopentene
E) 1-(1-hydroxycyclopentyl)-1-hydroxycyclopentane
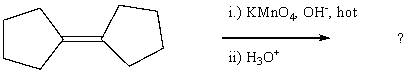
A) cyclopentanol
B) 1-cyclopentylcyclopentane
C) cyclopentanone
D) cyclopentene
E) 1-(1-hydroxycyclopentyl)-1-hydroxycyclopentane

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
59
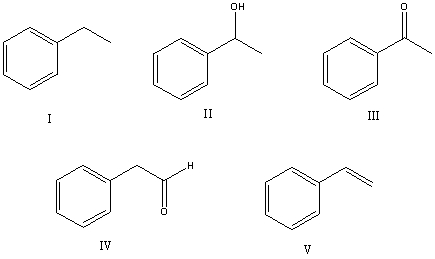
Select the structure of the major product in the following reaction. 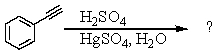
A) Ethylbenzene
B) 1-Phenylethanol
C) Acetophenone
D) 2-Phenylethanal
E) Vinylbenzene
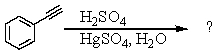
A) Ethylbenzene
B) 1-Phenylethanol
C) Acetophenone
D) 2-Phenylethanal
E) Vinylbenzene

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is not a synthesis of benzophenone, 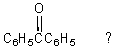
A)
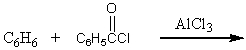
B)
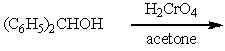
C)
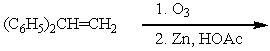
D)
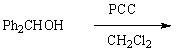
E) All of the above will give benzophenone.
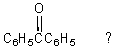
A)
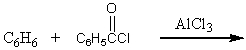
B)
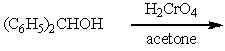
C)
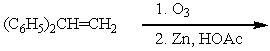
D)
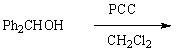
E) All of the above will give benzophenone.

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
61
Which compound is an acetal? 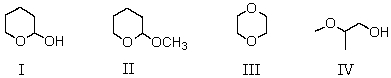
A) I
B) II
C) III
D) IV
E) All of the above
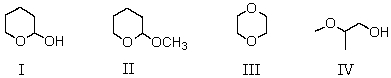
A) I
B) II
C) III
D) IV
E) All of the above

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
62
The product,D,of the following sequence of reactions 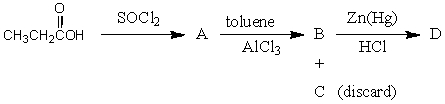 would be:
would be: 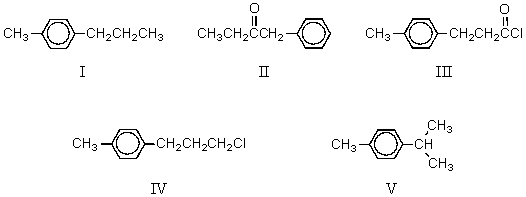
A) I
B) II
C) III
D) IV
E) V
 would be:
would be: 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
63
The product,E,of the following reaction sequence, 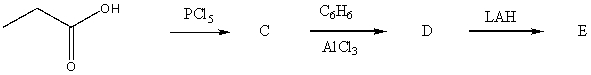 would be:
would be: 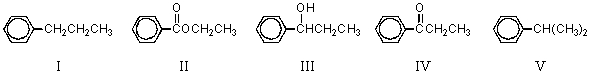
A) I
B) II
C) III
D) IV
E) V
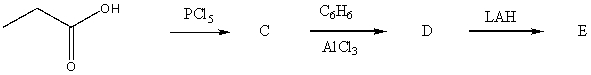 would be:
would be: 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
64
The product,K,of the following sequence of reactions 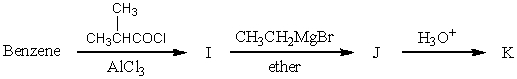 would be:
would be: 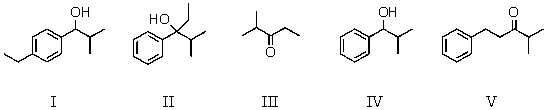
A) I
B) II
C) III
D) IV
E) V
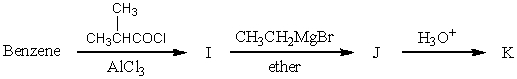 would be:
would be: 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
65
What,in general,is the order of increasing reactivity of these carbonyl compounds towards nucleophilic reagents? 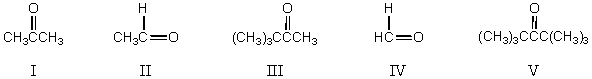
A) I < III < V < II < IV
B) IV < II < I < III < V
C) V< III < I < II < IV
D) II< I < V < III < IV
E) III < V < IV < II < I
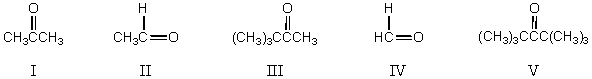
A) I < III < V < II < IV
B) IV < II < I < III < V
C) V< III < I < II < IV
D) II< I < V < III < IV
E) III < V < IV < II < I

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
66
The product,K,of the following sequence of reactions would be: 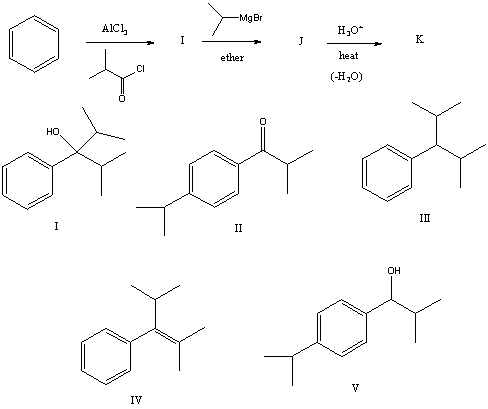
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
67
Which compound is a hemiacetal? 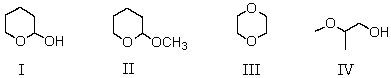
A) I
B) II
C) III
D) IV
E) All of the above
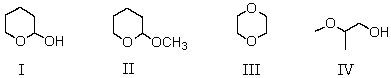
A) I
B) II
C) III
D) IV
E) All of the above

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
68
What is the final product of this synthetic sequence? 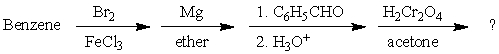
A)
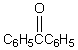
B) p-BrC6H4CH2C6H5
C) C6H5CH2COOH
D)
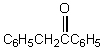
E) C6H5CH2C6H5
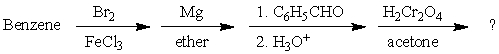
A)
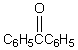
B) p-BrC6H4CH2C6H5
C) C6H5CH2COOH
D)
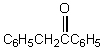
E) C6H5CH2C6H5

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
69
What would be the product of the following reaction sequence? 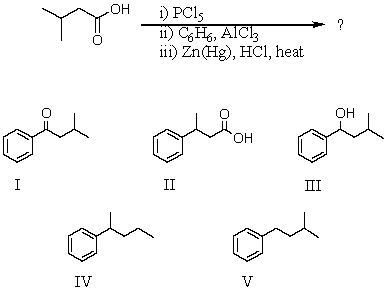
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
70
What would be the product of the following reaction sequence? 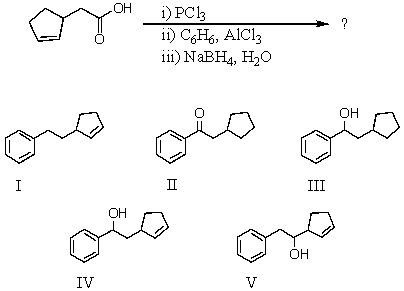
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
71
Which of these will not catalyze the reaction of a weak nucleophile with the carbonyl group of an aldehyde or ketone?
A) I-
B) H3O+
C) AlCl3
D) BF3
E) ZnCl2
A) I-
B) H3O+
C) AlCl3
D) BF3
E) ZnCl2

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following compounds is an acetal? 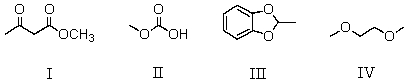
A) I
B) II
C) III
D) IV
E) None of these

A) I
B) II
C) III
D) IV
E) None of these

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
73
What new compound will eventually be formed when HCl is added to a solution of pentanal in methanol?
A)
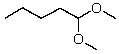
B)
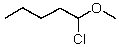
C)
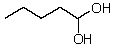
D)
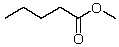
E)
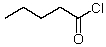
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
74
The product,E,of the following reaction sequence, 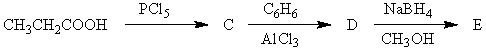 would be:
would be: 
A) I
B) II
C) III
D) IV
E) V
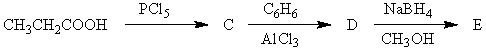 would be:
would be: 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
75
What would be the product of the following reaction sequence? 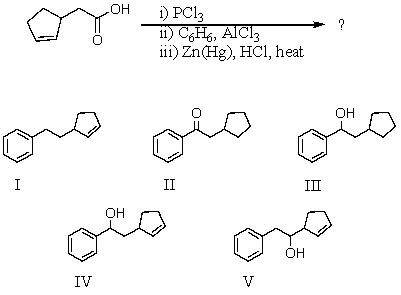
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
76
What would be the product of the following reaction sequence? 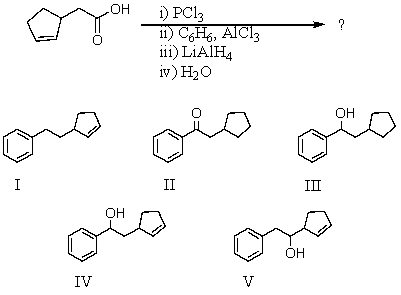
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
77
Acetals are unstable in the presence of an aqueous solution of which of these?
A) HCl
B) NaOH
C) KHCO3
D) Na2CO3
E) NaCl
A) HCl
B) NaOH
C) KHCO3
D) Na2CO3
E) NaCl

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
78
What would be the product of the following reaction sequence? 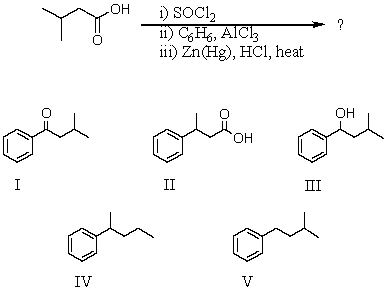
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
79
Which is the general formula for a thioacetal?
A)
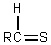
B)
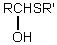
C)
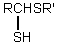
D)
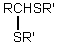
E)
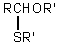
A)
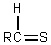
B)
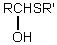
C)
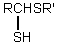
D)
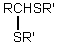
E)
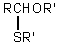

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck
80
What,in general,is the order of decreasing reactivity of these carbonyl compounds towards nucleophilic reagents? 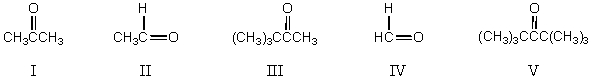
A) I > III > IV > II > V
B) IV > II > I > III > V
C) V > III > I > II > IV
D) I > IV > II > III > V
E) III > V > IV > II > I
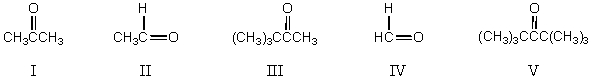
A) I > III > IV > II > V
B) IV > II > I > III > V
C) V > III > I > II > IV
D) I > IV > II > III > V
E) III > V > IV > II > I

Unlock Deck
Unlock for access to all 165 flashcards in this deck.
Unlock Deck
k this deck


