Deck 15: Reactions of Aromatic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/173
Play
Full screen (f)
Deck 15: Reactions of Aromatic Compounds
1
Which of these is a satisfactory synthesis of 1-chloro-2-phenylethane?
A) C6H5CH2CH3 + Cl2,Fe --
B) C6H5CH2CH3 + Cl2,400 C --
C) C6H5CH2CH2OH + PCl5 --
D) C6H5CH=CH2 + HCl,peroxide --
E) C6H6 + CH3CH2Cl,AlCl3 --
A) C6H5CH2CH3 + Cl2,Fe --
B) C6H5CH2CH3 + Cl2,400 C --
C) C6H5CH2CH2OH + PCl5 --
D) C6H5CH=CH2 + HCl,peroxide --
E) C6H6 + CH3CH2Cl,AlCl3 --
C6H5CH2CH2OH + PCl5 --
2
Which of these is a satisfactory synthesis of 1-chloro-2-phenylethane?
A) C6H5CH2CH3 + Cl2,Fe --
B) C6H5CH2CH3 + Cl2,400 C --
C) C6H5CH2CH2OH + SOCl2 --
D) C6H5CH=CH2 + HCl,peroxide --
E) C6H6 + CH3CH2Cl,AlCl3 --
A) C6H5CH2CH3 + Cl2,Fe --
B) C6H5CH2CH3 + Cl2,400 C --
C) C6H5CH2CH2OH + SOCl2 --
D) C6H5CH=CH2 + HCl,peroxide --
E) C6H6 + CH3CH2Cl,AlCl3 --
C6H5CH2CH2OH + SOCl2 --
3
Undesired polysubstitution of an aromatic nucleus is most likely to be encountered in the case of:
A) Friedel-Crafts alkylation
B) Friedel-Crafts acylation
C) Nitration
D) Sulfonation
E) Chlorination
A) Friedel-Crafts alkylation
B) Friedel-Crafts acylation
C) Nitration
D) Sulfonation
E) Chlorination
Friedel-Crafts alkylation
4
What is the chief product of the Friedel-Crafts alkylation of benzene with 1-butene and HF?
A) butylbenzene
B) 2-phenylbutane
C) 2-methyl-1-phenylpropane
D) t-butylbenzene
E) 2,2-diphenylbutane
A) butylbenzene
B) 2-phenylbutane
C) 2-methyl-1-phenylpropane
D) t-butylbenzene
E) 2,2-diphenylbutane

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following reaction: what is the expected product? 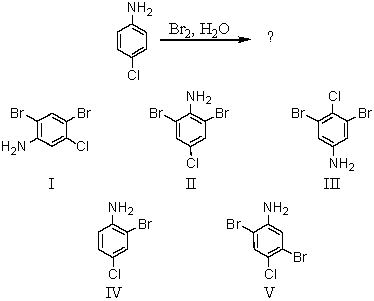
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
6
A mixture of chlorobenzene (1 mol)and acetanilide (1mol)is allowed to react with Br2 (0.5 mol)in the presence of trace amounts of FeBr3.What is the principal product of the competing reactions?
A) 1-bromo-4-chlorobenzene
B) 1-bromo-2-chlorobenzene
C) 1-bromo-3-chlorobenzene
D) 4-bromoacetanilide
E) 3-bromoacetanilide
A) 1-bromo-4-chlorobenzene
B) 1-bromo-2-chlorobenzene
C) 1-bromo-3-chlorobenzene
D) 4-bromoacetanilide
E) 3-bromoacetanilide

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
7
Which reagent would you use as the basis for a simple chemical test that would distinguish between toluene and vinylbenzene (C6H5CH=CH2)?
A) NaOH/H2O
B) Br2/CCl4
C) NaBH4
D) HCl/H2O
E) NaI in acetone
A) NaOH/H2O
B) Br2/CCl4
C) NaBH4
D) HCl/H2O
E) NaI in acetone

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
8
What is the chief product of the Friedel-Crafts alkylation of benzene with 1-butene and AlCl3?
A) butylbenzene
B) 2-phenylbutane
C) 2-methyl-1-phenylpropane
D) t-butylbenzene
E) 2,2-diphenylbutane
A) butylbenzene
B) 2-phenylbutane
C) 2-methyl-1-phenylpropane
D) t-butylbenzene
E) 2,2-diphenylbutane

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these is the rate-determining step in the nitration of benzene?
A) Protonation of nitric acid by sulfuric acid
B) Protonation of sulfuric acid by nitric acid
C) Loss of a water molecule by the protonated species to produce the nitronium ion
D) Addition of the nitronium to benzene to produce the arenium ion
E) Loss of a proton by the arenium ion to form nitrobenzene
A) Protonation of nitric acid by sulfuric acid
B) Protonation of sulfuric acid by nitric acid
C) Loss of a water molecule by the protonated species to produce the nitronium ion
D) Addition of the nitronium to benzene to produce the arenium ion
E) Loss of a proton by the arenium ion to form nitrobenzene

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
10
The electrophilic bromination or chlorination of benzene requires,in addition to the halogen:
A) a hydroxide ion.
B) a Lewis base.
C) a Lewis acid.
D) peroxide.
E) ultraviolet light.
A) a hydroxide ion.
B) a Lewis base.
C) a Lewis acid.
D) peroxide.
E) ultraviolet light.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following reactions would produce isopropylbenzene?
A)
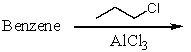
B)
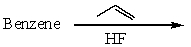
C)
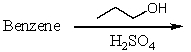
D)
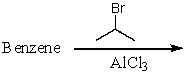
E) All of these
A)
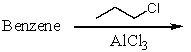
B)

C)

D)

E) All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following reactions could be used to synthesize tert-butylbenzene?
A)
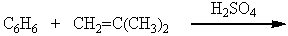
B)
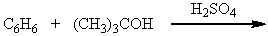
C)
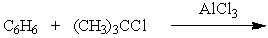
D) All of the above
E) None of the above
A)
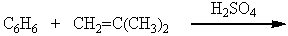
B)
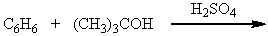
C)
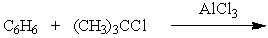
D) All of the above
E) None of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
13
This molecule does not normally participate as a reactant in a Friedel-Crafts reaction.
A) Benzene
B) Chlorobenzene
C) Nitrobenzene
D) Toluene
E) tert-Butylbenzene
A) Benzene
B) Chlorobenzene
C) Nitrobenzene
D) Toluene
E) tert-Butylbenzene

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following reactions would yield isopropylbenzene as the major product?
A)
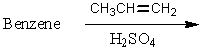
B)
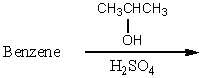
C)
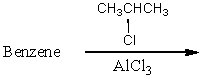
D)
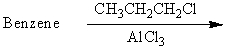
E) All of these
A)
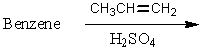
B)

C)
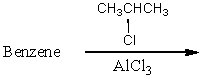
D)
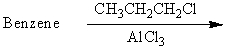
E) All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
15
In electrophilic aromatic substitution,the attacking species (the electrophile)necessarily is a:
A) neutral species.
B) positively charged species.
C) lewis acid.
D) proton.
E) carbocation.
A) neutral species.
B) positively charged species.
C) lewis acid.
D) proton.
E) carbocation.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of these molecules can be a reactant in a Friedel-Crafts reaction?
A) Aniline
B) Nitrobenzene
C) Chloroethene
D) Bromobenzene
E) p-Bromonitrobenzene
A) Aniline
B) Nitrobenzene
C) Chloroethene
D) Bromobenzene
E) p-Bromonitrobenzene

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
17
Which reagent or test could you use to distinguish between phenylacetylene and vinylbenzene?
A) NaOH/H2O
B) Br2/CCl4
C) IR Spectroscopy
D) CrO3/H2SO4
E) Concd.H2SO4
A) NaOH/H2O
B) Br2/CCl4
C) IR Spectroscopy
D) CrO3/H2SO4
E) Concd.H2SO4

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following reactions would produce cumene?
A)
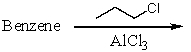
B)
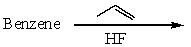
C)
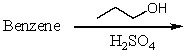
D)
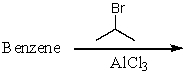
E) All of these
A)
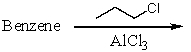
B)

C)

D)

E) All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these liquids would be unsuitable as an inert solvent for a Friedel-Crafts reaction?
A) Chlorobenzene
B) Nitrobenzene
C) Acetophenone
D) (Trifluoromethyl)benzene
E) All could be used.
A) Chlorobenzene
B) Nitrobenzene
C) Acetophenone
D) (Trifluoromethyl)benzene
E) All could be used.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following reactions would yield cumene as the major product?
A)
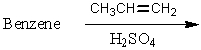
B)
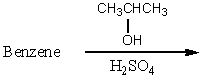
C)
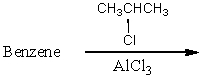
D)
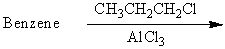
E) All of these
A)
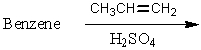
B)

C)
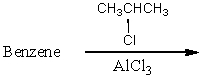
D)
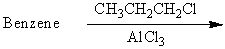
E) All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds would yield the greatest amount of meta product when subjected to ring nitration? 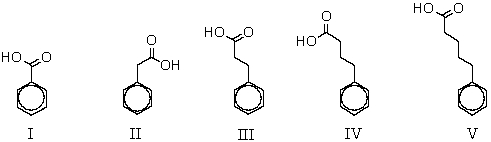
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is not an ortho-para director in electrophilic aromatic substitution?
A) -CF3
B) -OCH3
C) -CH3
D) -F
E) -NH2
A) -CF3
B) -OCH3
C) -CH3
D) -F
E) -NH2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
23
Which would be the product of the following reaction sequence? 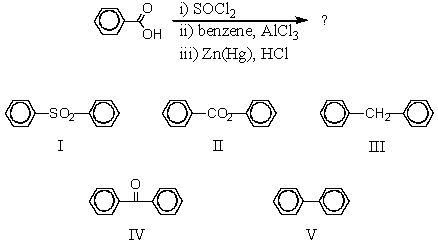
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
24
What would be the product of the following reaction sequence? 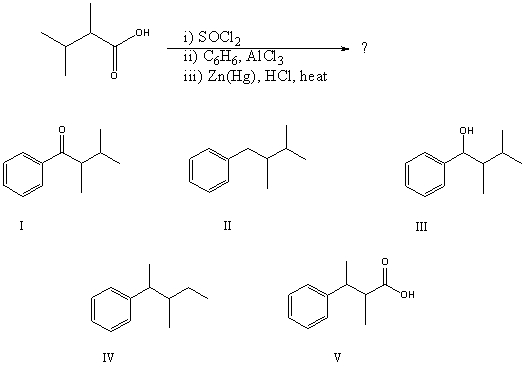
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
25
Which of these compounds gives essentially a single product on electrophilic substitution of a third group?
A) p-chlorotoluene
B) m-ethylanisole
C) 1-bromo-2-chlorobenzene
D) m-xylene
E) 3-hydroxybenzoic acid
A) p-chlorotoluene
B) m-ethylanisole
C) 1-bromo-2-chlorobenzene
D) m-xylene
E) 3-hydroxybenzoic acid

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
26
The reaction of benzene with (CH3)3CCH2Cl in the presence of anhydrous aluminum chloride produces principally which of these? 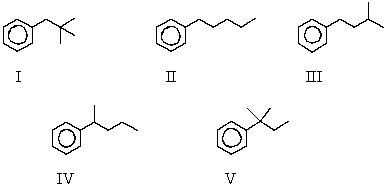
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
27
Arrange the following compounds in order of decreasing reactivity in electrophilic substitution: 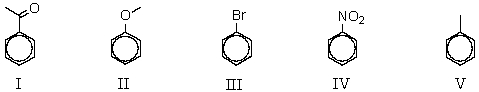
A) V > II > I > III > IV
B) II > V > III > I > IV
C) IV > I > III > V > II
D) III > II > I > IV > V
E) IV > V > II > I > III

A) V > II > I > III > IV
B) II > V > III > I > IV
C) IV > I > III > V > II
D) III > II > I > IV > V
E) IV > V > II > I > III

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is not a meta-directing substituent when present on the benzene ring?
A) -NHCOCH3
B) -NO2
C) -N(CH3)3+
D) -C N
E) -CO2H
A) -NHCOCH3
B) -NO2
C) -N(CH3)3+
D) -C N
E) -CO2H

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
29
What might be predicted to happen when the following substance undergoes Friedel-Crafts acylation? 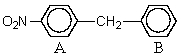
A) Substitution occurs in ring B,p- to the methylene group.
B) Substitution occurs in ring A,o- to the nitro group.
C) Substitution occurs in ring A,o- to the methylene group.
D) Substitution occurs in ring B,m- to the methylene group.
E) None of the above.No reaction will occur.

A) Substitution occurs in ring B,p- to the methylene group.
B) Substitution occurs in ring A,o- to the nitro group.
C) Substitution occurs in ring A,o- to the methylene group.
D) Substitution occurs in ring B,m- to the methylene group.
E) None of the above.No reaction will occur.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
30
What would you expect to be the major product obtained from the following reaction? 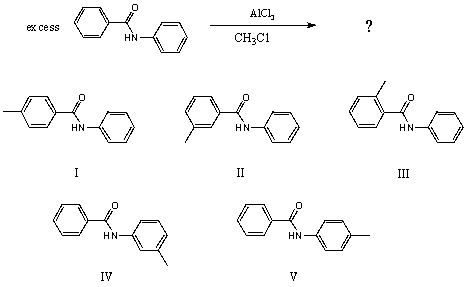
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
31
The reaction of benzene with 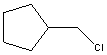 in the presence of anhydrous aluminum chloride produces principally which of these?
in the presence of anhydrous aluminum chloride produces principally which of these? 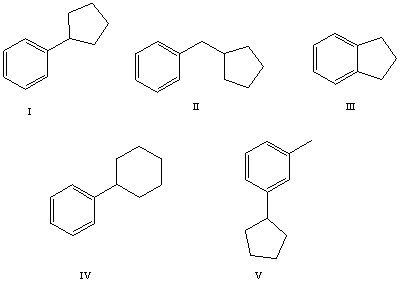
A) I
B) II
C) III
D) IV
E) V
 in the presence of anhydrous aluminum chloride produces principally which of these?
in the presence of anhydrous aluminum chloride produces principally which of these? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following reactions would give the product(s)indicated in substantial amounts (i.e. ,in greater than 50% yield)? 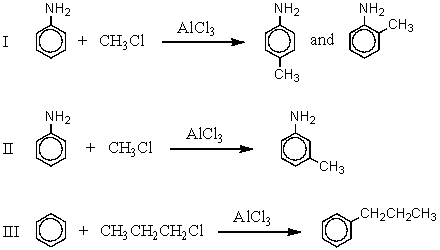
A) I
B) II
C) III
D) All of these
E) None of these

A) I
B) II
C) III
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
33
What would you expect to be the major product obtained from the following reaction? 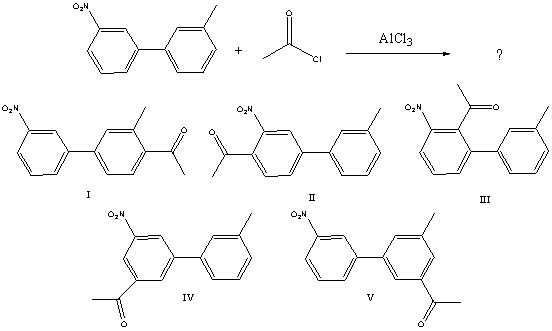
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
34
Which would be the product of the following reaction sequence? 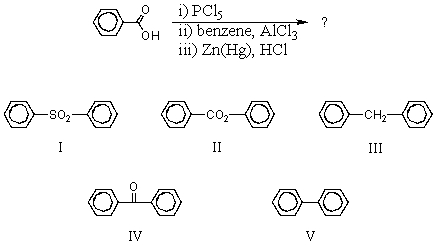
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
35
What would be the product of the following reaction sequence? 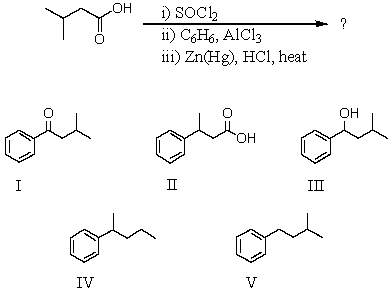
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
36
(Trifluoromethyl)benzene,C6H5CF3,will
A) nitrate rapidly in the ortho-para positions.
B) nitrate slowly in the ortho-para positions.
C) nitrate rapidly in the meta position.
D) nitrate slowly in the meta position.
E) fail to nitrate under any conditions.
A) nitrate rapidly in the ortho-para positions.
B) nitrate slowly in the ortho-para positions.
C) nitrate rapidly in the meta position.
D) nitrate slowly in the meta position.
E) fail to nitrate under any conditions.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
37
Which would be the product,X,of the following reaction sequence? 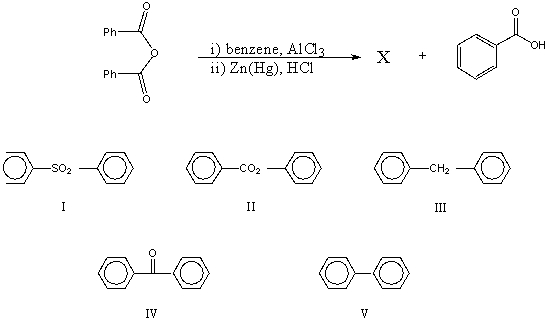
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
38
The major product(s)of the following reaction, 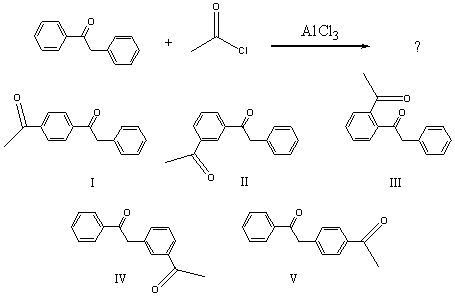 would be:
would be:
A) I
B) II
C) III
D) IV
E) V
 would be:
would be:A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
39
Which is the best prediction of the site(s)of substitution when 3-methylphenol is nitrated?
A) C-2
B) C-4
C) C-5
D) C-6
E) C-4 and C-6
A) C-2
B) C-4
C) C-5
D) C-6
E) C-4 and C-6

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction of benzene with 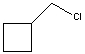 in the presence of anhydrous aluminum chloride produces principally which of these?
in the presence of anhydrous aluminum chloride produces principally which of these? 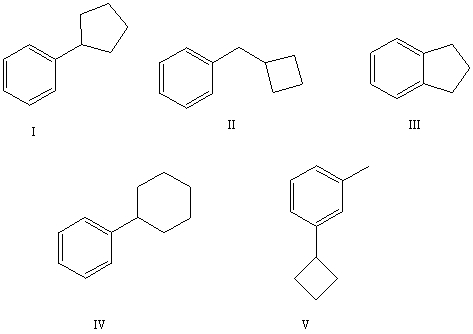
A) I
B) II
C) III
D) IV
E) V
 in the presence of anhydrous aluminum chloride produces principally which of these?
in the presence of anhydrous aluminum chloride produces principally which of these? 
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
41
Which reagent(s)would you use to carry out the following transformation? cumene -- 2- and 4-chlorocumene
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxides
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxides

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
42
What would you expect to be the major product obtained from the following reaction? 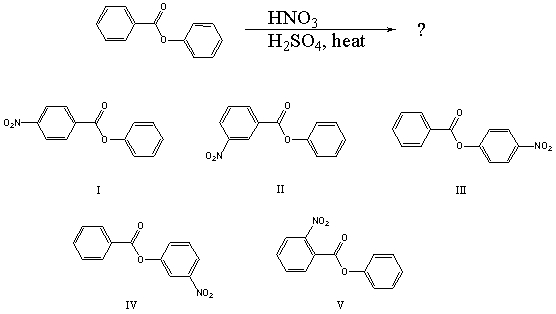
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
43
What would you expect to be the major product obtained from the following reaction? 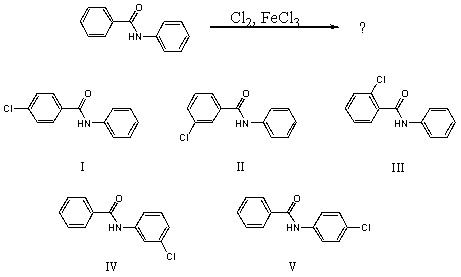
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
44
Which would be the major product(s)of the following reaction? 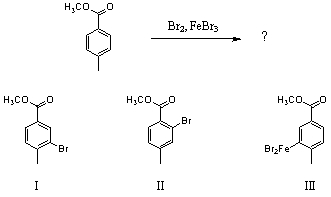
A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
45
What would you expect to be the major product obtained from the following reaction? 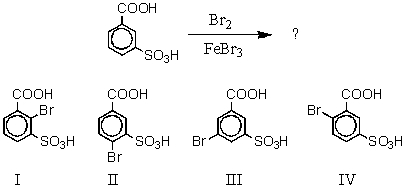
A) I
B) II
C) III
D) IV
E) Equal amounts of II and IV

A) I
B) II
C) III
D) IV
E) Equal amounts of II and IV

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
46
Which reagent(s)would you use to carry out the following transformation? isopropylbenzene -- 2- and 4-chloro-1-isopropylbenzene
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxides
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxides

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
47
What would you expect to be the major product obtained from the following reaction? 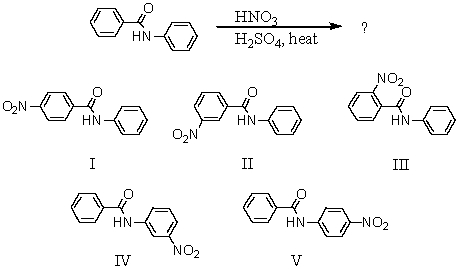
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
48
Which reagent(s)would you use to synthesize 2- and 4-bromo-1-cyclopentylbenzene from cyclopentylbenzene?
A) N-Bromosuccinimide (NBS),CCl4,light
B) PBr3
C) Br2,FeBr3
D) CH3CH2Br,AlBr3
E) HBr,ROOR
A) N-Bromosuccinimide (NBS),CCl4,light
B) PBr3
C) Br2,FeBr3
D) CH3CH2Br,AlBr3
E) HBr,ROOR

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
49
Which reagent(s)would you use to carry out the following transformation? ethylbenzene -- 2- and 4-chloro-1-ethylbenzene
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) None of these
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) None of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
50
What would you expect to be the major product obtained from the following reaction? 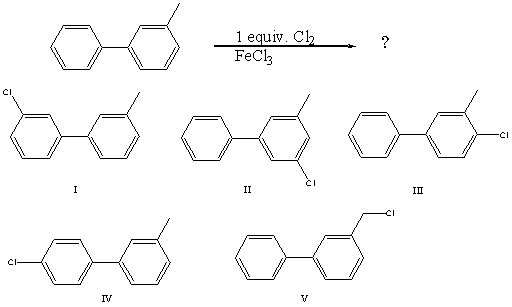
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
51
What would you expect to be the major product obtained from the following reaction? 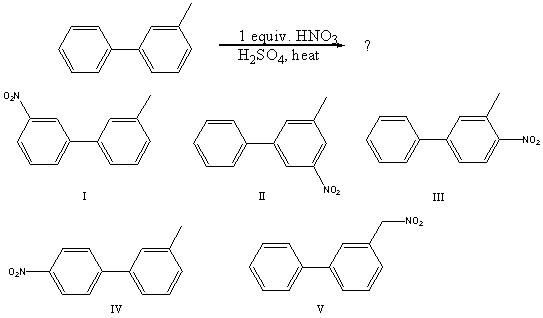
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
52
What would you expect to be the major product obtained from the monobromination of m-dichlorobenzene? 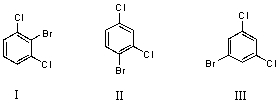
A) I
B) II
C) III
D) Equal amounts of I and II
E) Equal amounts of I,II and III

A) I
B) II
C) III
D) Equal amounts of I and II
E) Equal amounts of I,II and III

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
53
Which reagent(s)would you use to carry out the following transformation? t-butylbenzene -- p-chloro substitution product
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxide
A) Cl2,light,and heat
B) Cl2,FeCl3
C) SOCl2
D) C2H5Cl,AlCl3
E) HCl,peroxide

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
54
What would you expect to be the major product obtained from the following reaction? 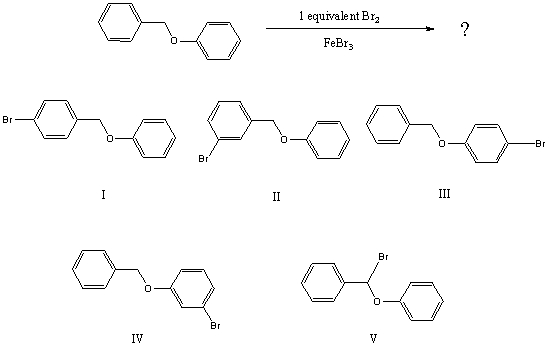
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
55
What would you expect to be the major product obtained from the following reaction? 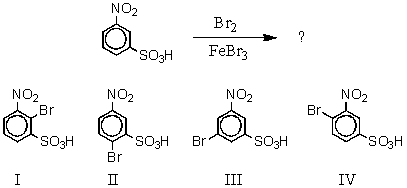
A) I
B) II
C) III
D) IV
E) Equal amounts of II and IV

A) I
B) II
C) III
D) IV
E) Equal amounts of II and IV

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
56
The major product(s),D,of the following reaction, 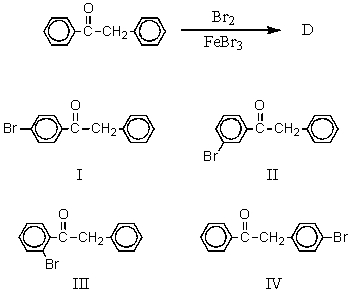 would be:
would be:
A) I
B) II
C) III
D) IV
E) Approximately equal amounts of I and II
 would be:
would be:A) I
B) II
C) III
D) IV
E) Approximately equal amounts of I and II

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
57
What would you expect to be the major product obtained from the following reaction? 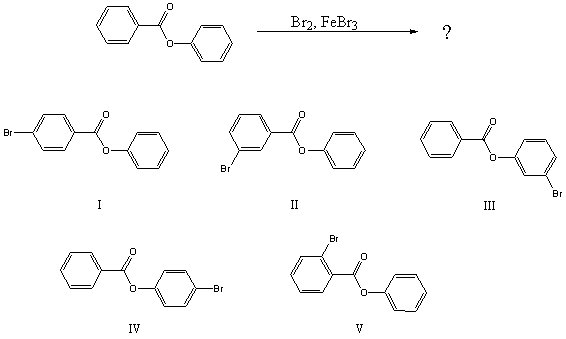
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
58
What would be the major product(s)of the following reaction? 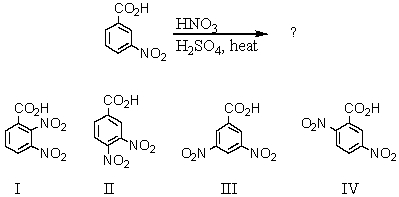
A) I
B) II
C) III
D) IV
E) Approximately equal amounts of II and IV

A) I
B) II
C) III
D) IV
E) Approximately equal amounts of II and IV

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
59
What would you expect to be the major product obtained from the following reaction? 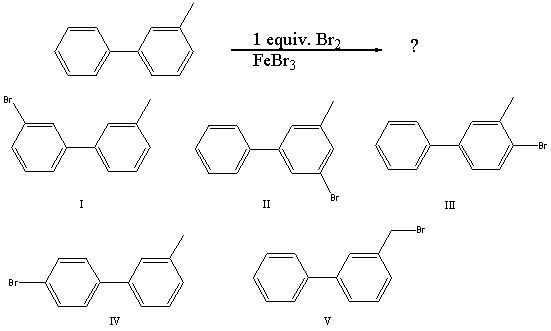
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
60
What would you expect to be the major product obtained from the following reaction? 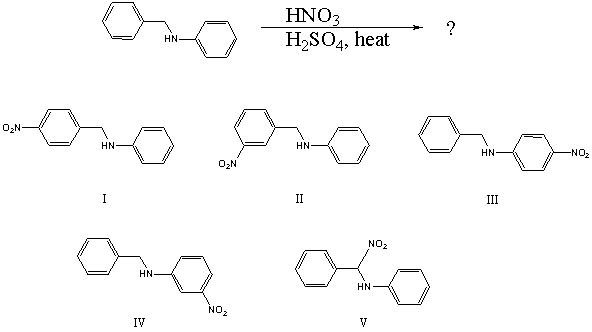
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds would be most reactive toward electrophilic substitution? 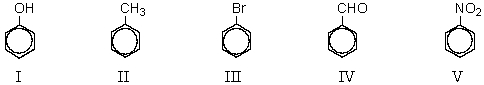
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
62
What would be the major product(s)of the following reaction? 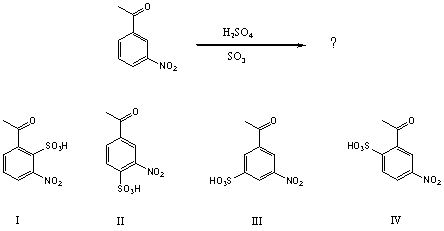
A) I
B) II
C) III
D) Equal amounts of I and IV
E) Equal amounts of II and IV

A) I
B) II
C) III
D) Equal amounts of I and IV
E) Equal amounts of II and IV

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
63
Which reagent would you use to carry out the following transformation? tert-butylbenzene
--
P-tert-butylbenzenesulfonic acid
+
O-tert-butylbenzenesulfonic acid
A) HNO3/H2SO4
B) tert-C4H9Cl/AlCl3
C) H2SO3/peroxides
D) SO3/H2SO4
E) SO2/H2SO3
--
P-tert-butylbenzenesulfonic acid
+
O-tert-butylbenzenesulfonic acid
A) HNO3/H2SO4
B) tert-C4H9Cl/AlCl3
C) H2SO3/peroxides
D) SO3/H2SO4
E) SO2/H2SO3

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following compounds would be least reactive toward electrophilic substitution? 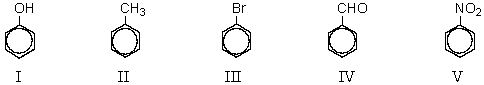
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following compounds would be most reactive to ring bromination? 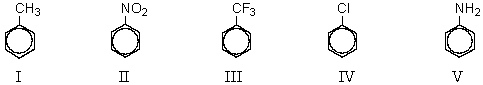
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
66
What would you expect to be the major product obtained from the following reaction? 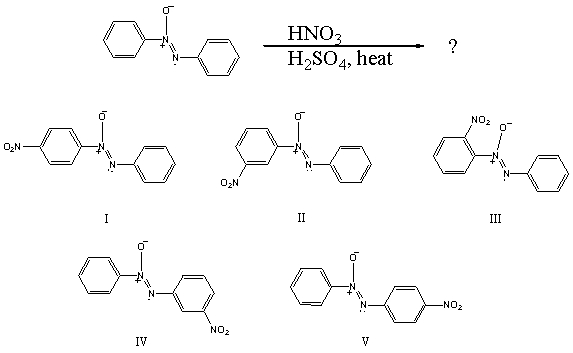
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
67
What would you expect to be the major product(s)obtained from the mononitration of m-dichlorobenzene? 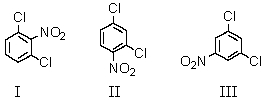
A) I
B) II
C) III
D) Equal amounts of I and II
E) Equal amounts of I,II and III

A) I
B) II
C) III
D) Equal amounts of I and II
E) Equal amounts of I,II and III

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following structures contribute(s)to the resonance hybrid of the intermediate formed when bromobenzene undergoes para-chlorination? 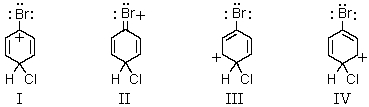
A) I
B) II
C) III
D) IV
E) All of the above

A) I
B) II
C) III
D) IV
E) All of the above

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds would be most reactive toward ring nitration? 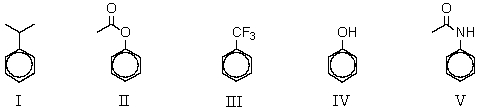
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following compounds would be most reactive toward ring bromination? 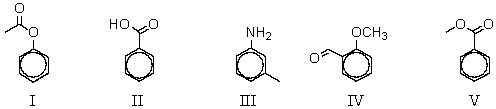
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the compounds listed below would you expect to give the greatest amount of meta-product when subjected to ring nitration? 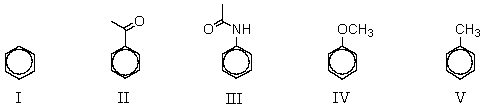
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
72
The major product(s),D,of the following reaction 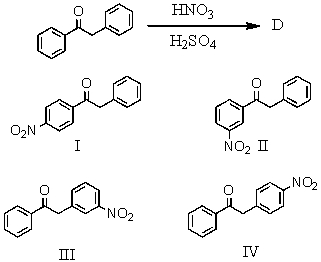 would be:
would be:
A) I
B) II
C) III
D) IV
E) Equal amounts of I and II
 would be:
would be:A) I
B) II
C) III
D) IV
E) Equal amounts of I and II

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the compounds listed below would you expect to give the greatest amount of meta-product when subjected to ring bromination? 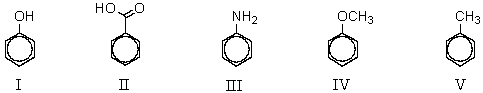
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
74
What would be the major product(s)of the following reaction? 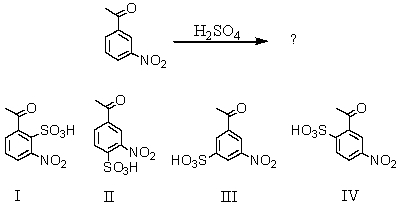
A) I
B) II
C) III
D) Equal amounts of I and IV
E) Equal amounts of II and IV

A) I
B) II
C) III
D) Equal amounts of I and IV
E) Equal amounts of II and IV

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
75
What would you expect to be the major product(s)obtained from the following reaction? 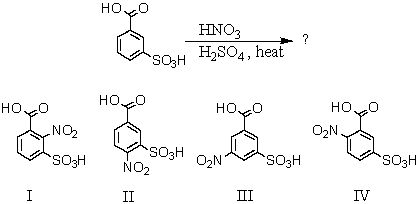
A) I
B) II
C) III
D) IV
E) Equal amounts of I and II

A) I
B) II
C) III
D) IV
E) Equal amounts of I and II

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
76
Which would be the major product(s)of the following reaction? 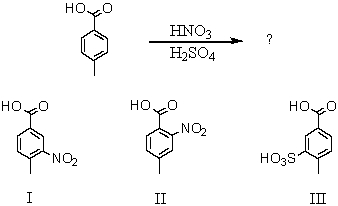
A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following compounds would be least reactive toward electrophilic substitution? 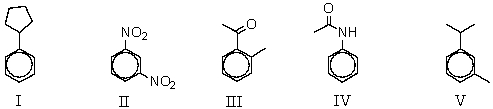
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
78
Which would be the major product(s)of the following reaction? 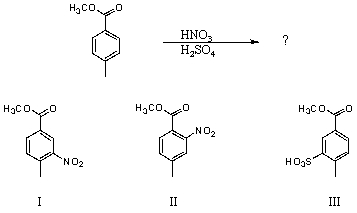
A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

A) I
B) II
C) III
D) I and II in roughly equal amounts
E) I and III in roughly equal amounts

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
79
The major product(s),C,of the following reaction, 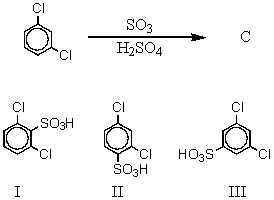 would be:
would be:
A) I
B) II
C) III
D) Approximately equal amounts of I and II
E) Approximately equal amounts of I and III
 would be:
would be:A) I
B) II
C) III
D) Approximately equal amounts of I and II
E) Approximately equal amounts of I and III

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following compounds would be most reactive toward electrophilic substitution? 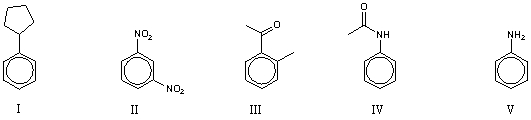
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck


