Deck 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/124
Play
Full screen (f)
Deck 18: Carboxylic Acids and Their Derivatives Nucleophilic Addition - Elimination at the Acyl Carbon
1
Which of the following is a keto-enol tautomeric pair? 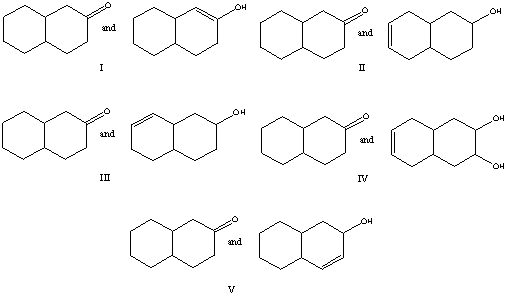
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
I
2
Which is the most acidic hydrogen in the compound shown? 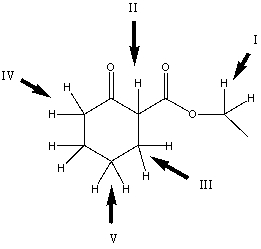
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
II
3
Which of the following is a keto-enol tautomeric pair? 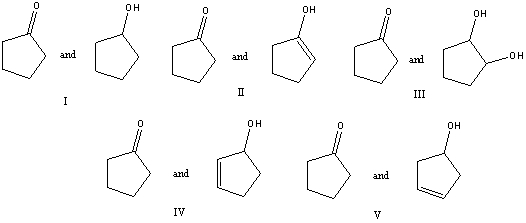
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V
II
4
Considering only the highlighted hydrogens,list the following compounds in order of increasing acidity: 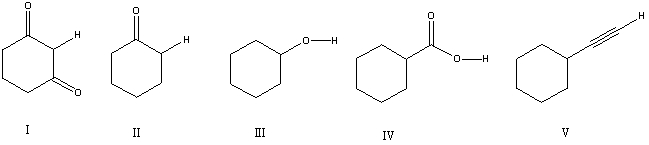
A) I,II,III,IV,V
B) II,V,III,I,IV
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,II,III,I,IV

A) I,II,III,IV,V
B) II,V,III,I,IV
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,II,III,I,IV

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
5
Which compound contains the most acidic hydrogens?
A) CH3CH2CH3
B) CH3CH=CH2
C) Cyclohexane
D) (CH3)2C=O
E) Benzene
A) CH3CH2CH3
B) CH3CH=CH2
C) Cyclohexane
D) (CH3)2C=O
E) Benzene

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
6
Which is the most acidic hydrogen in the compound shown? 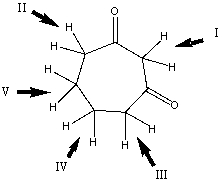
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a keto-enol tautomeric pair? 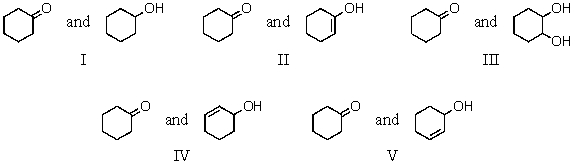
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following hydrogens is the most acidic? 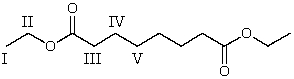
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these compounds would exist in an enol form to the greatest extent?
A)
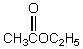
B)
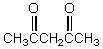
C)
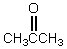
D)
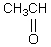
E)
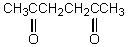
A)
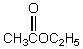
B)
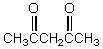
C)
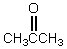
D)
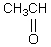
E)
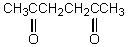

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds is the strongest acid? 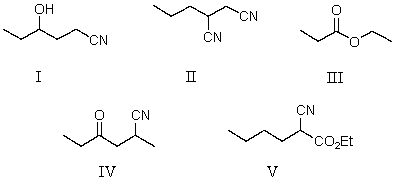
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following represent keto-enol tautomers?
A)
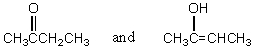
B)
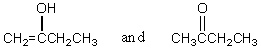
C)
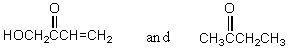
D) More than one of these
E) None of these
A)
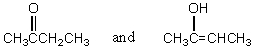
B)
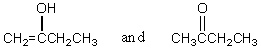
C)
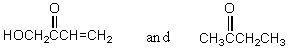
D) More than one of these
E) None of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
12
Considering only the highlighted hydrogens,list the following compounds in order of increasing acidity: 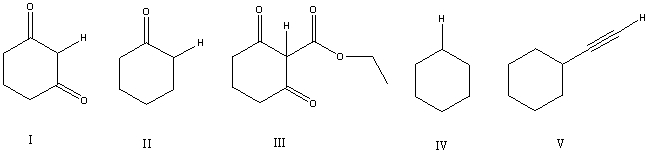
A) IV,II,III,I,V
B) II,V,IV,I,III
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

A) IV,II,III,I,V
B) II,V,IV,I,III
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
13
Which is the most acidic hydrogen in the compound shown? 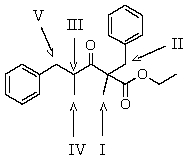
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the most acidic hydrogen in the compound shown? 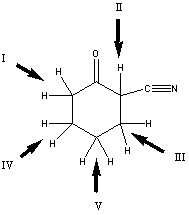
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
15
Considering only the highlighted hydrogens,list the following compounds in order of decreasing acidity: 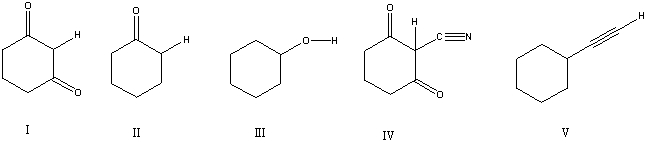
A) I,II,III,IV,V
B) II,V,III,I,IV
C) IV,I,III,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

A) I,II,III,IV,V
B) II,V,III,I,IV
C) IV,I,III,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
16
Considering only the highlighted hydrogens,list the following compounds in order of decreasing acidity: 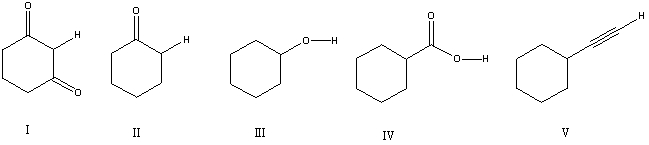
A) I,II,III,IV,V
B) II,V,III,I,IV
C) IV,I,III,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

A) I,II,III,IV,V
B) II,V,III,I,IV
C) IV,I,III,II,V
D) IV,V,II,I,III
E) V,IV,III,II,I

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following represent tautomers?
A)
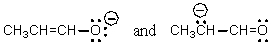
B)
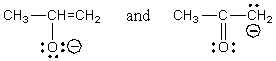
C)
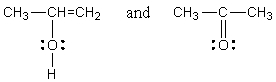
D) All of these
E) None of these
A)
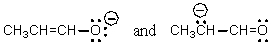
B)
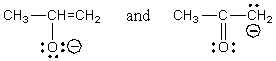
C)
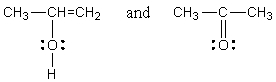
D) All of these
E) None of these

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
18
Considering only the highlighted hydrogens,list the following compounds in order of increasing acidity: 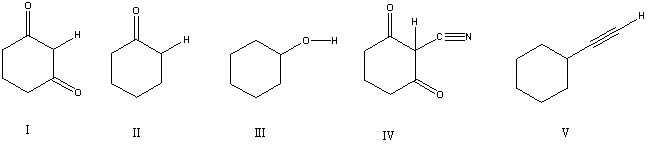
A) I,II,III,IV,V
B) II,V,III,I,IV
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,II,III,I,IV

A) I,II,III,IV,V
B) II,V,III,I,IV
C) III,I,IV,II,V
D) IV,V,II,I,III
E) V,II,III,I,IV

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
19
Considering only the highlighted hydrogens,list the following compounds in order of decreasing acidity: 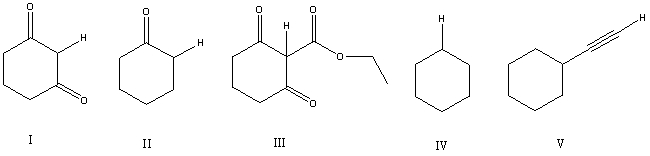
A) I,II,III,IV,V
B) III,V,IV,I,II
C) III,I,II,V,IV
D) IV,V,II,I,III
E) V,IV,III,II,I

A) I,II,III,IV,V
B) III,V,IV,I,II
C) III,I,II,V,IV
D) IV,V,II,I,III
E) V,IV,III,II,I

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
20
Which is the most acidic hydrogen in the compound shown? 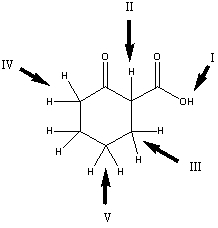
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
21
Explain why the following tautomer equilibrium lies far to the right. 


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would undergo racemization in base? 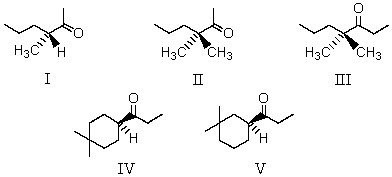
A) I
B) II and III
C) IV and V
D) I and V
E) I,IV and V

A) I
B) II and III
C) IV and V
D) I and V
E) I,IV and V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following would not undergo racemization in base?
A) (S)-2-phenylbutanal
B) (S)-3-phenylbutanal
C) (S)-3-phenyl-2-butanone
D) (S)-3-methyl-2-phenylbutanal
E) All of the above will undergo racemization in base.
A) (S)-2-phenylbutanal
B) (S)-3-phenylbutanal
C) (S)-3-phenyl-2-butanone
D) (S)-3-methyl-2-phenylbutanal
E) All of the above will undergo racemization in base.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
24
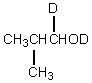
Which would be formed when 2-methylpropanal is dissolved in D2O containing NaOD?
A)
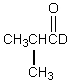
B)
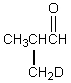
C)
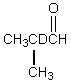
D)
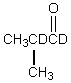
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
25
Which would be formed when 2-methylpropanal is dissolved in D2O containing D+?
A)
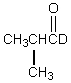
B)
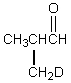
C)
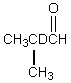
D)
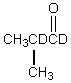
E)
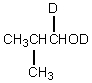
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
26
What would be the major product of the following reaction? 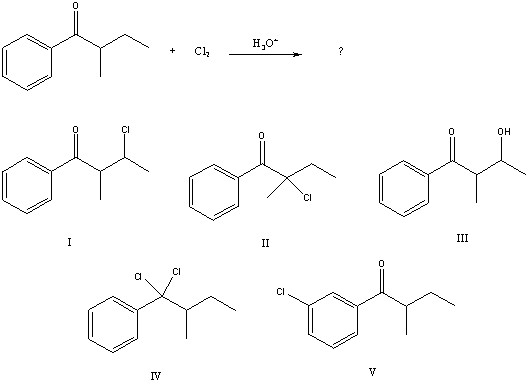
A) I
B) II
C) III
D) IV
E) V
B

A) I
B) II
C) III
D) IV
E) V
B

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following would undergo racemization in base? 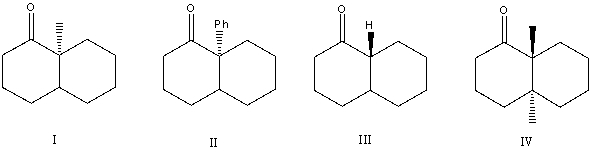
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
28
What synthetic strategy would accomplish the following transformation? 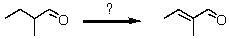
A) i)Br2,H3O+;ii)NaOC2H5,C2H5OH,heat
B) i)Cl2,FeCl3;ii)NaOC2H5,C2H5OH,heat
C) i)HCN;ii)H3O+,heat
D) i)Br2,h ;ii)(CH3)3COK, (CH3)3COH,heat
E) None of the above
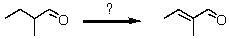
A) i)Br2,H3O+;ii)NaOC2H5,C2H5OH,heat
B) i)Cl2,FeCl3;ii)NaOC2H5,C2H5OH,heat
C) i)HCN;ii)H3O+,heat
D) i)Br2,h ;ii)(CH3)3COK, (CH3)3COH,heat
E) None of the above

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
29
Simple enols are less stable than the tautomeric keto forms because:
A) severe angle strain exists in the enol forms.
B) fewer atoms are coplanar in the keto form.
C) the enol cannot be chiral.
D) the C-C bond is weaker than the C-O bond.
E) Actually,simple enols are the more stable.
A) severe angle strain exists in the enol forms.
B) fewer atoms are coplanar in the keto form.
C) the enol cannot be chiral.
D) the C-C bond is weaker than the C-O bond.
E) Actually,simple enols are the more stable.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
30
What would be the major product of the following reaction? 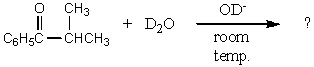
A)
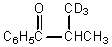
B)
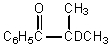
C)
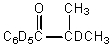
D)
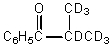
E)
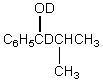
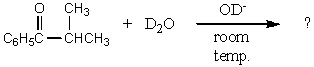
A)
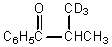
B)
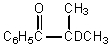
C)
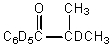
D)
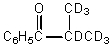
E)


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
31
What would be the major product of the following reaction? 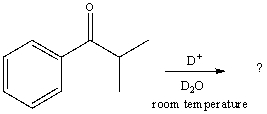
A)
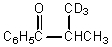
B)
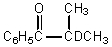
C)
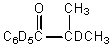
D)
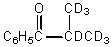
E)
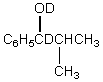

A)
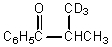
B)
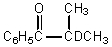
C)
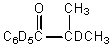
D)
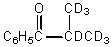
E)


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
32
What would be the major product of the following reaction? 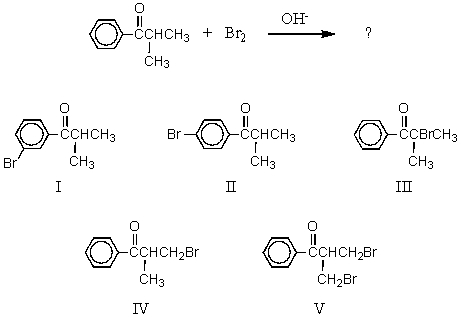
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following would undergo racemization in base? 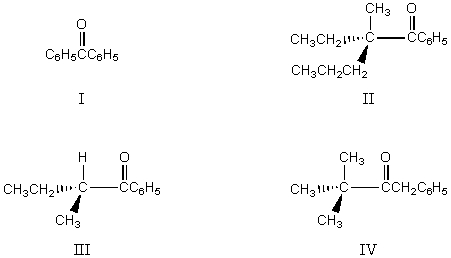
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
34
What would be the major product of the following reaction? 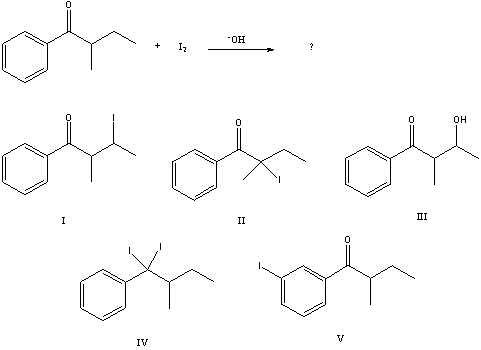
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
35
What would be the major product of the following reaction? 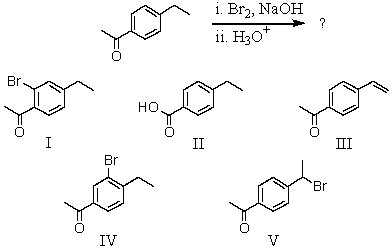
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following would not undergo racemization in base?
A) (R)-3-methyl-4-heptanone
B) (R)-3-methyl-2-heptanone
C) (R)-4-methyl-2-heptanone
D) (R)-2,4-dimethyl-3-heptanone
E) All of the above will undergo racemization in base.
A) (R)-3-methyl-4-heptanone
B) (R)-3-methyl-2-heptanone
C) (R)-4-methyl-2-heptanone
D) (R)-2,4-dimethyl-3-heptanone
E) All of the above will undergo racemization in base.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
37
What would be the major product of the following reaction? 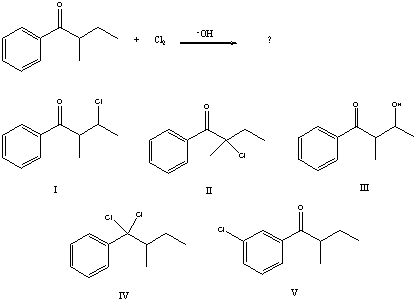
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
38
Which compound would be formed when 2-methylbutanal is treated with a solution of NaOD in D2O?
A)
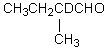
B)
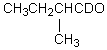
C)
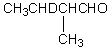
D)
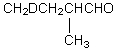
E)
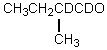
A)
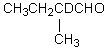
B)
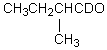
C)
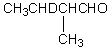
D)
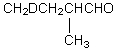
E)
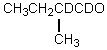

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
39
Explain why the following tautomer equilibrium lies far to the right. 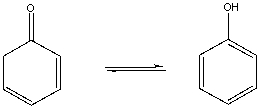


Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
40
Which compound would be formed when 2-methylbutanal is treated with a solution of D+ in D2O?
A)
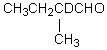
B)
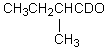
C)
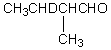
D)
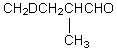
E)
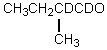
A)
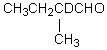
B)
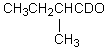
C)
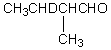
D)
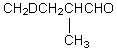
E)
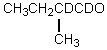

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
41
What would be the major product of the following reaction? 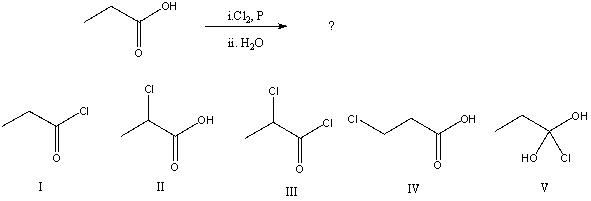
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
42
The conversion of 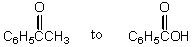 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?
A) Ag(NH3)2+
B) O3
C) NaOCl (Cl2 in NaOH)
D)
E) Cu++
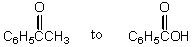 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?A) Ag(NH3)2+
B) O3
C) NaOCl (Cl2 in NaOH)
D)
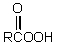
E) Cu++

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
43
What would be the major product of the following reaction? 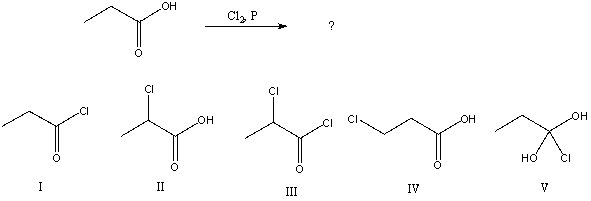
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
44
What would be the major product of the following reaction sequence? 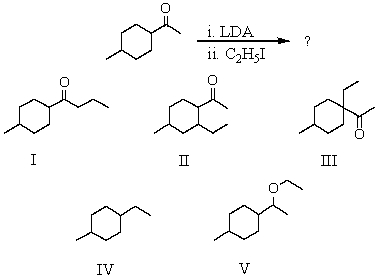
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
45
Which reagent would best serve as the basis for a simple chemical test to distinguish between CH3CHO and CH3COCH3?
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) C6H5NHNH2
D) NaHCO3/H2O
E) Ag(NH3)2+
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) C6H5NHNH2
D) NaHCO3/H2O
E) Ag(NH3)2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
46
A compound,X,C9H10O,which reacts with I2/NaOH to give a pale yellow precipitate,gives the following 1H NMR spectrum. Singlet,
2.0
Singlet,
3.0
Multiplet,
7.7
Which is a possible structure for X?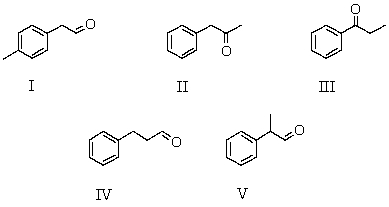
A) I
B) II
C) III
D) IV
E) V
2.0
Singlet,
3.0
Multiplet,
7.7
Which is a possible structure for X?

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the reagents listed below would serve as the basis for a simple chemical test to distinguish between 2-methyl-1-propanol and 2-butanol?
A) NaOI (I2 in NaOH)
B) KMnO4 in H2O
C) Br2 in CCl4
D) Cold concd H2SO4
E) CrO3 in H2SO4
A) NaOI (I2 in NaOH)
B) KMnO4 in H2O
C) Br2 in CCl4
D) Cold concd H2SO4
E) CrO3 in H2SO4

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
48
Which reagent would best serve as the basis for a simple chemical test to distinguish between 2-pentanone and 3-pentanone?
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) CrO3/H2SO4
D) NaHCO3/H2O
E) Ag(NH3)2+
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) CrO3/H2SO4
D) NaHCO3/H2O
E) Ag(NH3)2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
49
What would be the major product of the following reaction? 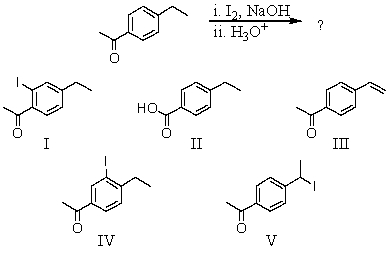
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
50
A compound,X,C9H10O,which reacts with I2/NaOH to give a pale yellow precipitate,gives the following 1H NMR spectrum. Singlet,
2.38
Singlet,
2.54
Multiplet, (centered)
7.50
Which is a possible structure for X?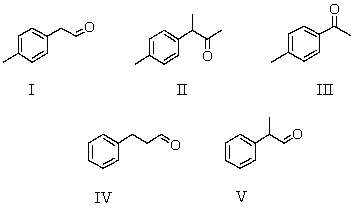
A) I
B) II
C) III
D) IV
E) V
2.38
Singlet,
2.54
Multiplet, (centered)
7.50
Which is a possible structure for X?

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
51
A compound,C5H10O,reacts with phenylhydrazine and gives a positive iodoform test.The compound could be which of these?
A)
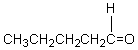
B)
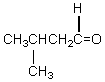
C) CH2=CHCH2CHOHCH3
D)
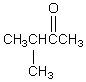
E)
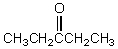
A)
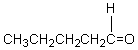
B)

C) CH2=CHCH2CHOHCH3
D)

E)
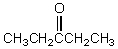

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
52
Which reagent would best serve as the basis for a simple chemical test to distinguish between 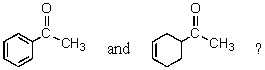
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) CrO3/H2SO4
D) NaHCO3/H2O
E) Ag(NH3)2+
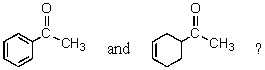
A) NaOI (I2 in NaOH)
B) Br2/CCl4
C) CrO3/H2SO4
D) NaHCO3/H2O
E) Ag(NH3)2+

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
53
The conversion of 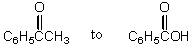 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?
A) Ag(NH3)2+
B) O3
C) NaOBr (Br2 in NaOH)
D)
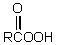
E) Cu++
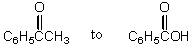 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?A) Ag(NH3)2+
B) O3
C) NaOBr (Br2 in NaOH)
D)
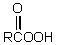
E) Cu++

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
54
What would be the major product of the following reaction? 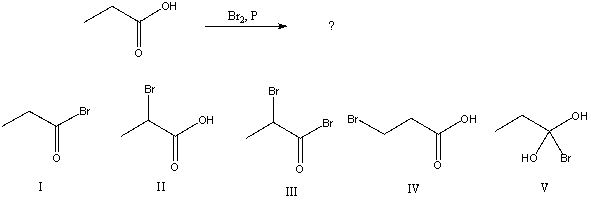
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
55
What would be the major product of the following reaction? 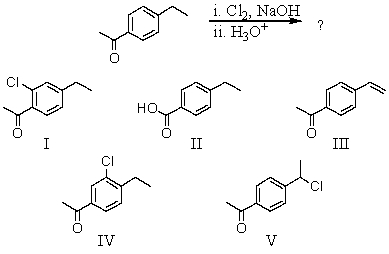
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
56
A negative iodoform test will be observed in the case of which of these?
A) Acetone
B) Ethanal
C) Ethanol
D) 2-Butanol
E) All of these will give a positive test.
A) Acetone
B) Ethanal
C) Ethanol
D) 2-Butanol
E) All of these will give a positive test.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
57
What would be the major product of the following reaction? 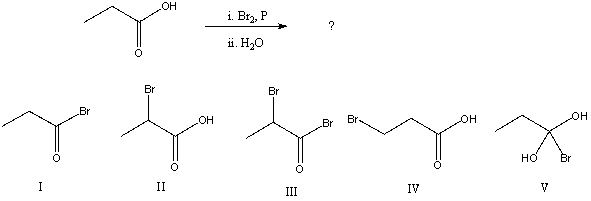
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
58
What would be the major product of the following reaction? 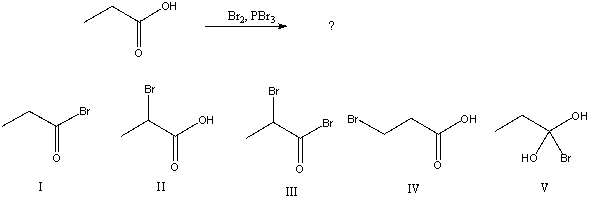
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
59
The conversion of 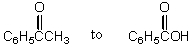 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?
A) Ag(NH3)2+
B) O3
C) NaOI (I2 in NaOH)
D)
E) Cu++
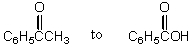 is accomplished by the use of which oxidizing agent?
is accomplished by the use of which oxidizing agent?A) Ag(NH3)2+
B) O3
C) NaOI (I2 in NaOH)
D)
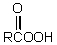
E) Cu++

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
60
Which compound could be subjected to a haloform reaction to produce m-chlorobenzoic acid? 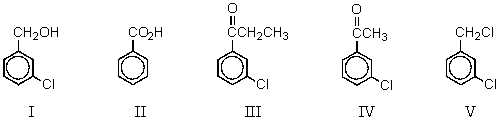
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the synthesis below.What is compound Z? 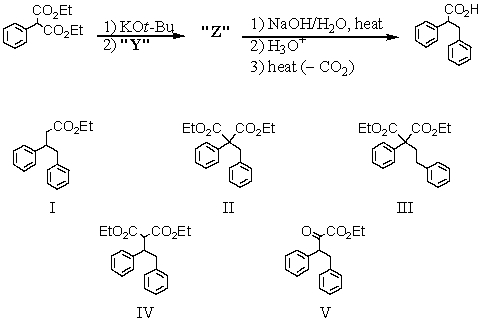
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
62
What is the product,W,of the following reaction sequence? 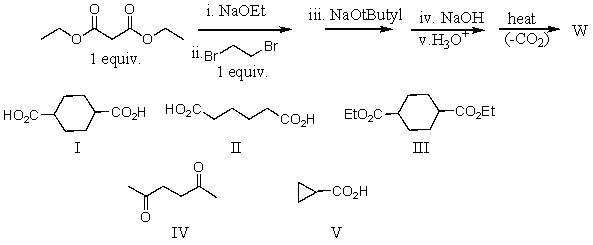
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following would provide the best synthesis of 3,5-dimethyl-2-hexanone?
A) Ethyl acetoacetate + NaOC2H5 + CH3I;then KO-t-Bu + (CH3)3CCH2Br;then NaOH;then H3O+;then heat
B) Ethyl acetoacetate + NaOC2H5 + (CH3)3CBr;then KO-t-Bu + CH3I;then NaOH;then H3O+;then heat
C) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2Br;then KO-t-Bu + CH3I;then NaOH;then H3O+;then heat
D) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CHBrCH3;then NaOH;then H3O+;then heat
E) Ethyl acetoacetate + NaOC2H5 + CH3I;then KO-t-Bu + (CH3)2CHBr;then NaOH;then H3O+;then heat
A) Ethyl acetoacetate + NaOC2H5 + CH3I;then KO-t-Bu + (CH3)3CCH2Br;then NaOH;then H3O+;then heat
B) Ethyl acetoacetate + NaOC2H5 + (CH3)3CBr;then KO-t-Bu + CH3I;then NaOH;then H3O+;then heat
C) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2Br;then KO-t-Bu + CH3I;then NaOH;then H3O+;then heat
D) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CHBrCH3;then NaOH;then H3O+;then heat
E) Ethyl acetoacetate + NaOC2H5 + CH3I;then KO-t-Bu + (CH3)2CHBr;then NaOH;then H3O+;then heat

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
64
What is the product,W,of the following reaction sequence? 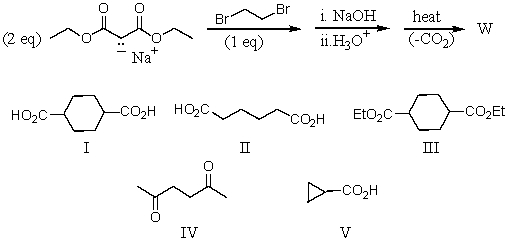
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the synthesis below.What is compound Z? 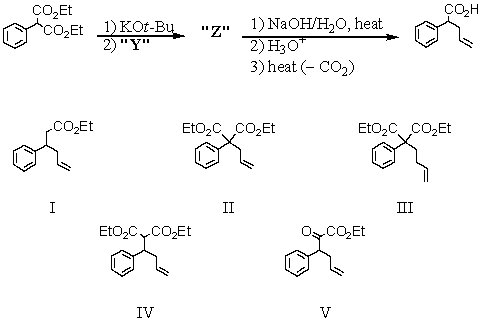
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
66
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 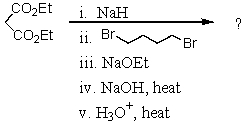
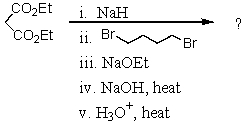

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
67
Which base is employed in the alkylation of methyl hexanoate with ethyl iodide?
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Sodium amide
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Sodium amide

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
68
What would be the major product of the following reaction sequence? 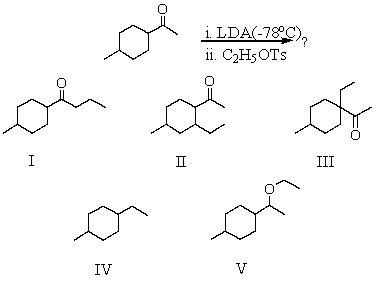
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
69
What is the product,L,of the following reaction sequence? 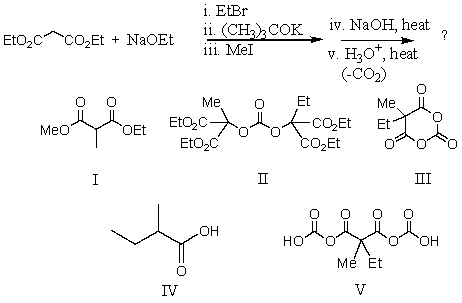
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
70
Which base is employed in the alkylation of methyl hexanoate with ethyl iodide?
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Lithium diisopropylamide
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Lithium diisopropylamide

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
71
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 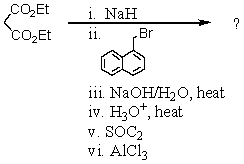
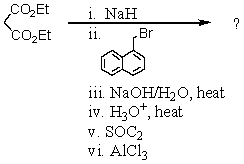

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
72
Which base is employed in the alkylation of ethyl pentanoate with methyl iodide?
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Lithium diisopropylamide
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Lithium diisopropylamide

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
73
What would be the product,P,of the following reaction sequence? 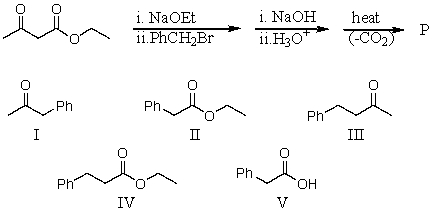
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
74
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 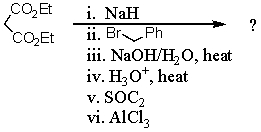
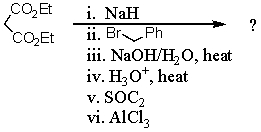

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
75
What would be the major product of the following reaction sequence? 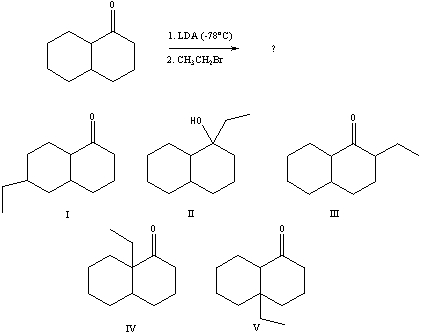
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
76
Which base is employed in the alkylation of ethyl pentanoate with methyl iodide?
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Sodium amide
A) Sodium methoxide
B) Sodium ethoxide
C) Sodium hydride
D) Potassium tert-butoxide
E) Sodium amide

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the synthesis below.What is compound Y? 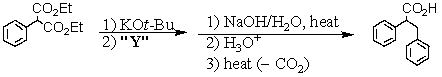
A) BrCH2CO2Et
B) EtO-CO-CO-OEt
C) CH3CH2Br
D) C6H5CH2Br
E) C6H5Br
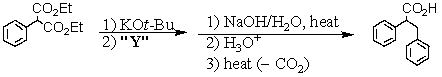
A) BrCH2CO2Et
B) EtO-CO-CO-OEt
C) CH3CH2Br
D) C6H5CH2Br
E) C6H5Br

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the product of the following reaction sequence. 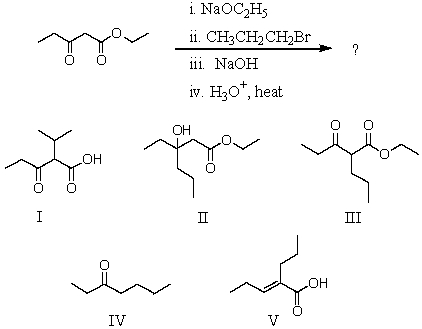
A) I
B) II
C) III
D) IV
E) V

A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
79
Which of these halides is predicted to alkylate malonic ester (as the anion)in highest yield?
A) CH3I
B) C6H5Br
C) (CH3)3CCH2Cl
D) CH3CHClCH3
E) All of these should be equally effective.
A) CH3I
B) C6H5Br
C) (CH3)3CCH2Cl
D) CH3CHClCH3
E) All of these should be equally effective.

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following would provide the best synthesis of 3-ethyl-6-methyl-2-heptanone?
A) Ethyl acetoacetate + NaOC2H5 + CH3CH2I;then KO-t-Bu + (CH3)3CCH2CH2Br;then NaOH;then H3O+;then heat
B) Ethyl acetoacetate + NaOC2H5 + (CH3)3CCH2Br;then KO-t-Bu + CH3CH2I;then NaOH;then H3O+;then heat
C) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CH2Br;then KO-t-Bu + CH3CH2I;then NaOH;then H3O+;then heat
D) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CH2CHBrCH2CH3;then NaOH;then H3O+;then heat
E) Ethyl acetoacetate + NaOC2H5 + CH3CH2I;then KO-t-Bu + (CH3)2CHCH2Br;then NaOH;then H3O+;then heat
A) Ethyl acetoacetate + NaOC2H5 + CH3CH2I;then KO-t-Bu + (CH3)3CCH2CH2Br;then NaOH;then H3O+;then heat
B) Ethyl acetoacetate + NaOC2H5 + (CH3)3CCH2Br;then KO-t-Bu + CH3CH2I;then NaOH;then H3O+;then heat
C) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CH2Br;then KO-t-Bu + CH3CH2I;then NaOH;then H3O+;then heat
D) Ethyl acetoacetate + NaOC2H5 + (CH3)2CHCH2CH2CHBrCH2CH3;then NaOH;then H3O+;then heat
E) Ethyl acetoacetate + NaOC2H5 + CH3CH2I;then KO-t-Bu + (CH3)2CHCH2Br;then NaOH;then H3O+;then heat

Unlock Deck
Unlock for access to all 124 flashcards in this deck.
Unlock Deck
k this deck


