Deck 18: Heat and the First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 18: Heat and the First Law of Thermodynamics
1
Glass beads of mass 100 g and specific heat 0.20 cal/g · Cº are heated to 90ºC and placed in a 300-g glass beaker containing 200 g of water at 20ºC.When equilibrium is reached,the temperature is approximately
A)23ºC
B)25ºC
C)27ºC
D)32ºC
E)39ºC
A)23ºC
B)25ºC
C)27ºC
D)32ºC
E)39ºC
25ºC
2
A lake with 8.0 109 kg of water,which has a specific heat of 4180 J/kg · Cº,warms from 10 to 15ºC.The amount of heat transferred to the lake is
A)2.5 103 J
B)1.7 1014 J
C)4.0 1015 J
D)1.7 1016 J
E)2.8 1016 J
A)2.5 103 J
B)1.7 1014 J
C)4.0 1015 J
D)1.7 1016 J
E)2.8 1016 J
1.7 1014 J
3
Two liquids,A and B,are mixed together.Liquid A has mass m and was initially at temperature 40 C,and liquid B has mass 2m and was initially at temperature 5 C.The specific heat of liquid A is 1.5 times that of liquid B. Calculate the final temperature of the mixture.
A)33.5 C
B)14.3 C
C)17.0 C
D)20.0 C
E)25.7 C
A)33.5 C
B)14.3 C
C)17.0 C
D)20.0 C
E)25.7 C
20.0 C
4
At liquid 4He temperatures (< 4.2 K),the molar heat capacity heat of most insulators goes as C = AT3,where A is a constant and T is in kelvins.The ratio of the heat required to raise the temperature from 2 K to 3K and from 1 K to 2K is
A)3/2
B)19/7
C)65/15
D)1/2
E)1/1
A)3/2
B)19/7
C)65/15
D)1/2
E)1/1

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
A 2.0-kg mass of iron (specific heat = 0.12 kcal/kg · Cº)at a temperature of 430ºC is dropped into 48 kg of water.The water is initially at a temperature of 10ºC.With no heat losses to the surroundings,the equilibrium temperature of the iron and water is approximately
A)12ºC
B)18ºC
C)19ºC
D)30ºC
E)33ºC
A)12ºC
B)18ºC
C)19ºC
D)30ºC
E)33ºC

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
A 250-g piece of lead is heated to 100ºC and is then placed in a 400-g copper container holding 500 g of water.The specific heat of copper is c = 0.386 kJ/kg · K.The container and the water had an initial temperature of 18.0ºC.When thermal equilibrium is reached,the final temperature of the system is 19.15ºC.If no heat has been lost from the system,what is the specific heat of the lead? (the specific heat of water is 4.180 kJ/kg · K)
A)0.119 kJ/kg · K
B)0.128 kJ/kg · K
C)0.110 kJ/kg · K
D)0.0866 kJ/kg · K
E)0.0372 kJ/kg · K
A)0.119 kJ/kg · K
B)0.128 kJ/kg · K
C)0.110 kJ/kg · K
D)0.0866 kJ/kg · K
E)0.0372 kJ/kg · K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Aluminum has a specific heat more than twice that of copper.Identical masses of aluminum and copper,both at 0ºC,are dropped together into a can of hot water.When the system has come to equilibrium,
A)the aluminum is at a higher temperature than the copper.
B)the copper is at a higher temperature than the aluminum.
C)the aluminum and copper are at the same temperature.
D)the difference in temperature between the aluminum and the copper depends on the amount of water in the can.
E)the difference in temperature between the aluminum and the copper depends on the initial temperature of the water in the can.
A)the aluminum is at a higher temperature than the copper.
B)the copper is at a higher temperature than the aluminum.
C)the aluminum and copper are at the same temperature.
D)the difference in temperature between the aluminum and the copper depends on the amount of water in the can.
E)the difference in temperature between the aluminum and the copper depends on the initial temperature of the water in the can.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The quantity of heat absorbed by a body is determined from the formula Q = cm(Tf - Ti).A certain metal has a specific heat c = 0.21 cal/g Cº and a mass m = 25.6 g.The initial temperature is Ti = 34.6ºC,and the final temperature Tf = 54.6ºC.The quantity of heat absorbed is
A)+23 cal
B)+0.23 cal
C)+14 cal
D)+110 cal
E)+207 cal
A)+23 cal
B)+0.23 cal
C)+14 cal
D)+110 cal
E)+207 cal

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Two liquids,A and B,are mixed together,and the resulting temperature is 22 C.If liquid A has mass m and was initially at temperature 35 C,and liquid B has mass 3m and was initially at temperature 11 C,calculate the ratio of the specific heats of A divided by B.
A)0.85
B)2.5
C)1.2
D)0.45
E)0.94
A)0.85
B)2.5
C)1.2
D)0.45
E)0.94

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Body A has twice the mass and three times the specific heat of body B.They are supplied with equal amounts of heat.Body A experiences a temperature change T.What change in temperature is experienced by body B?
A) T
B)3 T/2
C)2 T/3
D)6 T
E) T/2
A) T
B)3 T/2
C)2 T/3
D)6 T
E) T/2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about heat and work is the proper usage of the terms?
A)a system has 50 J of heat
B)50 J of heat is transferred from the environment to the system
C)a system has 50 J of work
D)a system does 50 J of work on the environment
E)(B)and (D)
A)a system has 50 J of heat
B)50 J of heat is transferred from the environment to the system
C)a system has 50 J of work
D)a system does 50 J of work on the environment
E)(B)and (D)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
Use the following to answer question: 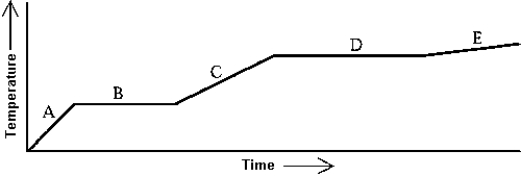
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the liquid can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the liquid can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The molar specific heat of copper is 24.5 J/(mol.K).The amount of heat needed to raise 126 g of copper by 2C is
A)24.5 J
B)49 J
C)12.3 J
D)98 J
E)147 J
A)24.5 J
B)49 J
C)12.3 J
D)98 J
E)147 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
For most metals,the heat capacity goes as C = T + AT3 at low temperatures (~ 100 K),whereas insulators only has the T3 term.Both and A are constants.The two terms in the expression for C suggests
A)there are at least two degrees of freedom in metals.
B)there are two independent mechanisms for heat to be absorbed or released in metals.
C)that there are two kinds of heat that are absorbed by metals.
D)that thermal energy is transferred to metals differently than insulators.
E)nothing.It just happens that way.
A)there are at least two degrees of freedom in metals.
B)there are two independent mechanisms for heat to be absorbed or released in metals.
C)that there are two kinds of heat that are absorbed by metals.
D)that thermal energy is transferred to metals differently than insulators.
E)nothing.It just happens that way.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
The specific heat of a substance is a measure of
A)energy needed to change the kinetic energies of atoms and/or molecules and/or electrons by a temperature increment.
B)total energy in the substance for a given temperature change.
C)energy needed to vaporize/liquefy the substance.
D)energy needed to liquefy/solidify the substance.
E)none of the above
A)energy needed to change the kinetic energies of atoms and/or molecules and/or electrons by a temperature increment.
B)total energy in the substance for a given temperature change.
C)energy needed to vaporize/liquefy the substance.
D)energy needed to liquefy/solidify the substance.
E)none of the above

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
A 1.0-kg piece of marble at 100ºC is dropped into 2.5 kg of water at 1.0ºC and the resulting temperature is 7.0ºC.The specific heat of the marble is approximately
A)0.16 kcal/kg · Cº
B)0.75 kcal/kg · Cº
C)0.61 kcal/kg · Cº
D)0.30 kcal/kg · Cº
E)0.26 kcal/kg · Cº
A)0.16 kcal/kg · Cº
B)0.75 kcal/kg · Cº
C)0.61 kcal/kg · Cº
D)0.30 kcal/kg · Cº
E)0.26 kcal/kg · Cº

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
To raise the temperature of a 2.0-kg piece of metal from 20º to 100ºC,61.8 kJ of heat is added.What is the specific heat of this metal?
A)0.39 kJ/kg · K
B)0.31 kJ/kg · K
C)1.6 kJ/kg · K
D)1.2 kJ/kg · K
E)0.77 kJ/kg · K
A)0.39 kJ/kg · K
B)0.31 kJ/kg · K
C)1.6 kJ/kg · K
D)1.2 kJ/kg · K
E)0.77 kJ/kg · K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
Use the following to answer question: 
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the solid can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The specific heat of the solid can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
A system has a heat capacity of 100 J.This means
A)it is possible to extract the 100 J of heat and convert it to work.
B)it is possible to transfer the 100 J of heat to the environment.
C)some of the heat capacity can be converted to work.
D)some of the heat capacity can be transferred to another system if there is a temperature difference.
E)(C)and (D)
A)it is possible to extract the 100 J of heat and convert it to work.
B)it is possible to transfer the 100 J of heat to the environment.
C)some of the heat capacity can be converted to work.
D)some of the heat capacity can be transferred to another system if there is a temperature difference.
E)(C)and (D)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
Use the following to answer question: 
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated at constant volume.This process is shown in the graph.The specific heat at constant volume of the vapor can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated at constant volume.This process is shown in the graph.The specific heat at constant volume of the vapor can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)dividing the rate at which heat is added by the product of the slope of A and the mass of the substance.
D)dividing the rate at which heat is added by the product of the slope of C and the mass of the substance.
E)dividing the rate at which heat is added by the product of the slope of E and the mass of the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
A 4-kg mass of metal of unknown specific heat at a temperature of 600 C is dropped into 0.5 kg of ice and 0.5 kg of water both at 0 C.With no heat losses to the surroundings,the equilibrium temperature of the mixture is 85 C.Calculate the specific heat of the metal.
A)0.04 kcal/kg C
B)0.06 kcal/kg C
C)0.08 kcal/kg C
D)1.6 kcal/kg C
E)1.2 kcal/kg C
A)0.04 kcal/kg C
B)0.06 kcal/kg C
C)0.08 kcal/kg C
D)1.6 kcal/kg C
E)1.2 kcal/kg C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
A small lake has a surface area of 10000 m2.Assuming that the average depth of the lake is 2 m,how much heat is released when the average temperature of the water in the lake drops by 1C ?
A)8.36 1010 J
B)8.36 107 J
C)2.0 108 J
D)2.0 1011 J
E)8.36 103 J
A)8.36 1010 J
B)8.36 107 J
C)2.0 108 J
D)2.0 1011 J
E)8.36 103 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
A container contains a 200 mL of 100% proof alcohol (i.e.,it has 50% ethyl alcohol and 50% water by volume)at the boiling point of the alcohol.How long does it take to distill (boil)all the alcohol if heat is supplied at a rate of 100 W? (The density and latent heat of vaporization of ethyl alcohol are 0.81 g/cm3,and 879 kJ/kg.)
A)712 s
B)1110 s
C)450 s
D)1450 s
E)950 s
A)712 s
B)1110 s
C)450 s
D)1450 s
E)950 s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
You add 50 g of ice cubes to 125 g of water that is initially at 20ºC in a calorimeter of negligible heat capacity.When the system has reached equilibrium,how much of the ice remains?
A)31 g
B)48 g
C)19 g
D)47 g
E)all of the ice melts
A)31 g
B)48 g
C)19 g
D)47 g
E)all of the ice melts

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
Use the following to answer question: 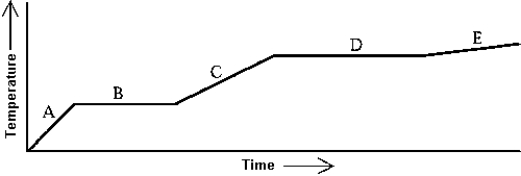
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.Which of the following statements is correct?
A)The latent heat of fusion is greater than the latent heat of vaporization.
B)The latent heat of vaporization is greater than the latent heat of fusion.
C)The latent heat of vaporization is equal to the latent heat of fusion.
D)The mass of the substance must be known before any statements about the latent heats can be made.
E)The relative sizes of the latent heats depend on the rate at which the heat is added.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.Which of the following statements is correct?
A)The latent heat of fusion is greater than the latent heat of vaporization.
B)The latent heat of vaporization is greater than the latent heat of fusion.
C)The latent heat of vaporization is equal to the latent heat of fusion.
D)The mass of the substance must be known before any statements about the latent heats can be made.
E)The relative sizes of the latent heats depend on the rate at which the heat is added.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
When a substance changes phase,from solid to liquid or liquid to vapor and vice versa,there is no change in temperature,even though heat is being added or removed.Why is there no change in temperature?
A)During a phase change,energy is used to break/establish the intermolecular bonds rather than stored as kinetic energy of the molecules.
B)During a phase change,temperature is not well defined since there are two phases involved.
C)During a phase change,energy is needed to overcome the gravitational pull.
D)During a phase change,the temperature measured is the last temperature of the measuring device.
E)none of the above
A)During a phase change,energy is used to break/establish the intermolecular bonds rather than stored as kinetic energy of the molecules.
B)During a phase change,temperature is not well defined since there are two phases involved.
C)During a phase change,energy is needed to overcome the gravitational pull.
D)During a phase change,the temperature measured is the last temperature of the measuring device.
E)none of the above

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
The specific heat of a gas at constant pressure is
A)directly proportional to the pressure.
B)inversely proportional to the pressure.
C)always greater than the specific heat at constant volume.
D)always less than the specific heat at constant volume.
E)independent of the kind of gas.
A)directly proportional to the pressure.
B)inversely proportional to the pressure.
C)always greater than the specific heat at constant volume.
D)always less than the specific heat at constant volume.
E)independent of the kind of gas.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
A group of explorers in the Antarctic can obtain the water they need only by melting snow.How much heat does it take for them to make a cup of coffee (100 g water at 100ºC)? Assume that the snow has an initial temperature of -40ºC; that the latent heats of fusion and vaporization of water are,respectively,333.5 kJ/kg and 2257 kJ/kg; and that the specific heats of ice (snow)and water are,respectively,2.05 kJ/kg · K and 4.18 kJ/kg · K.
A)33 kJ
B)50 kJ
C)75 kJ
D)83 kJ
E)310 kJ
A)33 kJ
B)50 kJ
C)75 kJ
D)83 kJ
E)310 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Use the following to answer question: 
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of fusion can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)multiplying the slope of A by the rate at which heat is added,and dividing by the mass of the substance.
D)multiplying the slope of C by the rate at which heat is added,and dividing by the mass of the substance.
E)multiplying the slope of E by the rate at which heat is added,and dividing by the mass of the substance.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of fusion can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)multiplying the slope of A by the rate at which heat is added,and dividing by the mass of the substance.
D)multiplying the slope of C by the rate at which heat is added,and dividing by the mass of the substance.
E)multiplying the slope of E by the rate at which heat is added,and dividing by the mass of the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
If the heat capacities of both ice and steam are 0.5 cal/g · Cº,the quantity of heat required to change 1 g of ice at -10ºC to steam at 120ºC is approximately
A)750 cal
B)735 cal
C)630 cal
D)620 cal
E)555 cal
A)750 cal
B)735 cal
C)630 cal
D)620 cal
E)555 cal

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
The temperature of water in the Gulf of Mexico can rise by about 20 F with the onset of the summer.However,the most significant temperature rise is confined to the top 2-3 meters of water.Assuming that it takes 45 days for the temperature to increase by 20 F,what is the average energy absorbed per day by water in a volume measuring 1 m 1 m 2.5 m?
A)1.16 109 J
B)2.58 106 J
C)4.64 107 J
D)2.09 109 J
E)2.5 105 J
A)1.16 109 J
B)2.58 106 J
C)4.64 107 J
D)2.09 109 J
E)2.5 105 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
A 3-kg mass of metal of specific heat = 0.1 kcal/kg C at a temperature of 600 C is dropped into 1.0 kg water at 20 C.With no heat losses to the surroundings,determine the equilibrium temperature of the mixture,and if it is 100 C,calculate what mass of water is turned into steam at this temperature.
A)100 C and 110 g of steam
B)100 C and 150 g of steam
C)100 C and 130 g of steam
D)100 C and 70 g of steam
E)The equilibrium temperature is not 100 C.
A)100 C and 110 g of steam
B)100 C and 150 g of steam
C)100 C and 130 g of steam
D)100 C and 70 g of steam
E)The equilibrium temperature is not 100 C.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
If the heat given off by 300 g of an alloy as it cools through 50 Cº is sufficient to raise the temperature of 300 g of water from 30º to 40ºC,the specific heat of the alloy must be approximately
A)0.015 cal/g · Cº
B)0.10 cal/g · Cº
C)0.15 cal/g · Cº
D)0.20 cal/g · Cº
E)0.50 cal/g · Cº
A)0.015 cal/g · Cº
B)0.10 cal/g · Cº
C)0.15 cal/g · Cº
D)0.20 cal/g · Cº
E)0.50 cal/g · Cº

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
The specific heat of a gas is
A)the same for all gases.
B)directly proportional to the absolute temperature.
C)independent of constraints imposed on it while heating.
D)a negligible quantity.
E)greater at constant pressure than at constant volume.
A)the same for all gases.
B)directly proportional to the absolute temperature.
C)independent of constraints imposed on it while heating.
D)a negligible quantity.
E)greater at constant pressure than at constant volume.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
A 2.0-kg mass of iron (specific heat = 0.12 kcal/kg C)at a temperature of 430 C is dropped into 0.4 kg of ice and 0.4 kg of water both at 0 C.With no heat losses to the surroundings,the equilibrium temperature of the mixture is approximately
A)0 C
B)100 C
C)23 C
D)69 C
E)87 C
A)0 C
B)100 C
C)23 C
D)69 C
E)87 C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
A container contains a 200 mL of 100% proof alcohol (i.e.,it has 50% ethyl alcohol and 50% water by volume)at 20 C.How much heat is needed to bring the mixture to the boiling point of the alcohol? (assume that the specific heat in the 100% proof can be treated as due to the alcohol and water separately,and the density,boiling point and specific heat of alcohol are 0.81 g/cm3,78 C,and 2.4 J/(g.C ),respectively)
A)32650 J
B)35525 J
C)11136 J
D)17400 J
E)48557 J
A)32650 J
B)35525 J
C)11136 J
D)17400 J
E)48557 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Use the following to answer question: 
Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of vaporization can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)multiplying the slope of A by the rate at which heat is added,and dividing by the mass of the substance.
D)multiplying the slope of C by the rate at which heat is added,and dividing by the mass of the substance.
E)multiplying the slope of E by the rate at which heat is added,and dividing by the mass of the substance.

Heat is added to a substance at a constant rate.The substance starts as a solid and is melted; the liquid is heated and vaporized; finally,the vapor is heated.This process is shown in the graph.The latent heat of vaporization can be found by
A)multiplying the length of B (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
B)multiplying the length of D (in seconds)by the rate at which heat is added,and dividing by the mass of the substance.
C)multiplying the slope of A by the rate at which heat is added,and dividing by the mass of the substance.
D)multiplying the slope of C by the rate at which heat is added,and dividing by the mass of the substance.
E)multiplying the slope of E by the rate at which heat is added,and dividing by the mass of the substance.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
If 100 g of steam at 100 C were mixed with 10 kg of ice at -100 C,find the final temperature of the mixture assuming no heat losses to the surroundings.
A)-85 C
B)-65 C
C)0 C
D)-15 C
E)-43 C
A)-85 C
B)-65 C
C)0 C
D)-15 C
E)-43 C

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
Some water is poured into some ice.What will happen to the water? Assume no heat loss to the surrounding.
A)The water stays as water.
B)Some of the water freezes.
C)All the water freezes.
D)Unable to tell what will happen.
E)It depends on how fast you pour the water into the ice.
A)The water stays as water.
B)Some of the water freezes.
C)All the water freezes.
D)Unable to tell what will happen.
E)It depends on how fast you pour the water into the ice.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
On a hot summer day,water collects on the outside of a glass of ice lemonade.The water comes from
A)inside the glass since glass is porous.
B)the condensation of the water vapor due the fact that the glass is much colder than the air.
C)the straw you use to drink your lemonade.
D)the mixture of water and lemonade.
E)It is one of the mysteries of life.
A)inside the glass since glass is porous.
B)the condensation of the water vapor due the fact that the glass is much colder than the air.
C)the straw you use to drink your lemonade.
D)the mixture of water and lemonade.
E)It is one of the mysteries of life.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Use the following to answer the question: 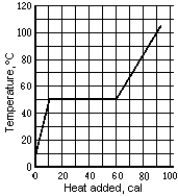
The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The melting point temperature is
A)10ºC
B)100ºC
C)60ºC
D)73ºC
E)None of these is correct.

The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The melting point temperature is
A)10ºC
B)100ºC
C)60ºC
D)73ºC
E)None of these is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Suppose you do 75 kJ of work on a system consisting of 10 kg of water by stirring it with a paddle wheel.During this process,40 kcal of heat is removed.The change in the internal energy of the system is
A)-35 kJ
B)-115 kJ
C)-134 kJ
D)-242 kJ
E)-156 kJ
A)-35 kJ
B)-115 kJ
C)-134 kJ
D)-242 kJ
E)-156 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
Use the following to answer the question: 
The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the liquid phase is
A)0.84 cal/g · Cº
B)0.25 cal/g · Cº
C)1.6 cal/g · Cº
D)1.7 cal/g · Cº
E)None of these is correct.

The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the liquid phase is
A)0.84 cal/g · Cº
B)0.25 cal/g · Cº
C)1.6 cal/g · Cº
D)1.7 cal/g · Cº
E)None of these is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
In a certain thermodynamic process,1000 cal of heat are added to a gas confined in a cylinder.At the same time,1000 J of work are done by the gas as it expands.The increase in internal energy of the gas is
A)zero
B)3186 J
C)-239 J
D)5186 J
E)1239 J
A)zero
B)3186 J
C)-239 J
D)5186 J
E)1239 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
Besides Joule's classic experiment,another way of demonstrating the equivalence between mechanical energy and heat is the following: Put some lead shot into a glass tube,seal both ends of the tube,invert the tube quickly several times,and measure the temperature of the shot.If you assume that all the mechanical energy is converted into heat in the lead shot and that no heat is lost,what is the change in the temperature of the shot if the tube is 1.0 m long,there are 100 g of shot,and the tube is inverted 10 times? (The specific heat of lead is 0.128 kJ/kg · K.)
A)0.77 Cº
B)0.077 Cº
C)2.5 Cº
D)7.7 Cº
E)0.25 Cº
A)0.77 Cº
B)0.077 Cº
C)2.5 Cº
D)7.7 Cº
E)0.25 Cº

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
The first law of thermodynamics has as its basis the same fundamental principle as
A)the continuity principle.
B)conservation of energy
C)Newton's law of universal gravitation.
D)static equilibrium.
E)the conservation of linear momentum.
A)the continuity principle.
B)conservation of energy
C)Newton's law of universal gravitation.
D)static equilibrium.
E)the conservation of linear momentum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
A small water reactor recently installed at Podunk College is operating at the boiling point of water due to the malfunctioning of the cooling system.The operators observe that the water boils away at the rate of 10 L/min.If they assume that all of the reactor energy is absorbed in the water,the power developed by the reactor is approximately
A)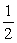 hp
hp
B)378 W
C)56 kW
D)378 kW
E)22.7 MW
A)
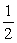 hp
hpB)378 W
C)56 kW
D)378 kW
E)22.7 MW

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
Use the following to answer the question: 
The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the solid phase is
A)0.6 cal/g · Cº
B)0.25 cal/g · Cº
C)1.6 cal/g · Cº
D)1.7 cal/g · Cº
E)None of these is correct.

The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The specific heat of the solid phase is
A)0.6 cal/g · Cº
B)0.25 cal/g · Cº
C)1.6 cal/g · Cº
D)1.7 cal/g · Cº
E)None of these is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
A 6.0-g lead bullet traveling at 300 m/s penetrates a wooden block and stops.If 50 percent of the initial kinetic energy of the bullet is converted into thermal energy in the bullet,by how much does the bullet's temperature increase? (The specific heat of lead is 128 J/kg · K.)
A)0.17º C
B)1.8 102 ºC
C)17 ºC
D)3.5 102 ºC
E)35 ºC
A)0.17º C
B)1.8 102 ºC
C)17 ºC
D)3.5 102 ºC
E)35 ºC

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
The first law of thermodynamics is most closely related to
A)the definition of absolute zero.
B)the definition of an ideal gas.
C)the conservation of energy.
D)thermal expansion.
E)the conservation of momentum.
A)the definition of absolute zero.
B)the definition of an ideal gas.
C)the conservation of energy.
D)thermal expansion.
E)the conservation of momentum.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
When a substance goes directly from a solid state to a gaseous form,the process is known as
A)vaporization.
B)evaporization.
C)condensation.
D)sublimation.
E)deposition.
A)vaporization.
B)evaporization.
C)condensation.
D)sublimation.
E)deposition.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
In a certain thermodynamic process,20 cal of heat are removed from a system and 30 cal of work are done on the system.The internal energy of the system
A)increases by 10 cal.
B)decreases by 10 cal.
C)increases by 50 cal.
D)decreases by 50 cal.
E)decreases by 20 cal.
A)increases by 10 cal.
B)decreases by 10 cal.
C)increases by 50 cal.
D)decreases by 50 cal.
E)decreases by 20 cal.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Two containers of equal volume are connected by a stopcock as shown below.One container is filled with a gas at a pressure of 1 atm and temperature of 293 K while the other container is evacuated so that it is under vacuum.The containers are thermally isolated from the surrounding so no heat enters or escaped from the system.The stopcock is then opened allowing the gas from one container to fill the other.What is the final temperature of the gas after it has come to equilibrium? 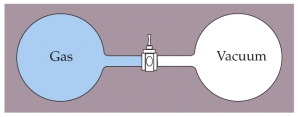
A)136.5 K
B)273 K
C)293 K
D)195 K
E)undetermined

A)136.5 K
B)273 K
C)293 K
D)195 K
E)undetermined

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
The percentage of mechanical energy that can theoretically be turned into heat energy according to the first law of thermodynamics is
A)100%
B)90%
C)75%
D)50%
E)0%
A)100%
B)90%
C)75%
D)50%
E)0%

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
A state variable is one that allows other variables to be determined using a relationship.Which of the following variables are state variables?
A)P,V,and T
B)Internal energy,U
C)W and Q
D)(A)and (B)
E)(A),(B),and (C)
A)P,V,and T
B)Internal energy,U
C)W and Q
D)(A)and (B)
E)(A),(B),and (C)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
How much internal energy is contained in 1 mole of monatomic gas at STP?
A)zero
B)1.11 kJ
C)2.22 kJ
D)3.33 kJ
E)5.55 kJ
A)zero
B)1.11 kJ
C)2.22 kJ
D)3.33 kJ
E)5.55 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
A system absorbs heat Q and has an equal amount of positive work done on it.What is the change in the internal energy of the system?
A)Q
B)2Q
C)-2Q
D)zero
E)Q/2
A)Q
B)2Q
C)-2Q
D)zero
E)Q/2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
In a certain process,500 cal of heat are supplied to a system consisting of a gas confined in a cylinder.At the same time,500 J of work are done by the gas by expansion.The increase in thermal energy of the gas is approximately
A)zero
B)1.00 kJ
C)1.59 kJ
D)2.09 kJ
E)2.59 kJ
A)zero
B)1.00 kJ
C)1.59 kJ
D)2.09 kJ
E)2.59 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
During a certain thermodynamic process,418 J of work are done on a system and 214 cal of heat are transferred to the system.The change in internal energy during the process is
A)314 cal
B)114 cal
C)468 cal
D)368 cal
E)632 cal
A)314 cal
B)114 cal
C)468 cal
D)368 cal
E)632 cal

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Use the following to answer the question: 
The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The heat of fusion of the material is
A)10 cal/g
B)50 cal/g
C)30 cal/g
D)90 cal/g
E)None of these is correct.

The graph shows the temperature of a 1.0-g sample of material as heat is added to it.The material is initially a solid at 10ºC.The pressure remains constant,and there is no chemical change.The heat of fusion of the material is
A)10 cal/g
B)50 cal/g
C)30 cal/g
D)90 cal/g
E)None of these is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
A system is said to go through an isothermal process if it
A)remains at a constant temperature.
B)does no work on its surroundings.
C)remains in the same state.
D)neither gains nor loses heat.
E)gains or loses heat at a constant rate.
A)remains at a constant temperature.
B)does no work on its surroundings.
C)remains in the same state.
D)neither gains nor loses heat.
E)gains or loses heat at a constant rate.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
A balloon contains gas at a pressure 1.2 atm (1 atm = 101.3 kPa)and has a volume of 0.10m3.More gas is pumped into the balloon at constant pressure until the volume is doubled.How much work is done by the pump?
A)12 J
B)24 kJ
C)24 J
D)12 kJ
E)6.1 kJ
A)12 J
B)24 kJ
C)24 J
D)12 kJ
E)6.1 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
Use the following to answer the question: 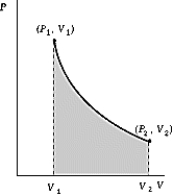
-One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 3 atm and a temperature of 30ºC to a pressure P2 = 1 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)50.3 kJ
B)63.5 kJ
C)95.9 kJ
D)131 kJ
E)158 kJ

-One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 3 atm and a temperature of 30ºC to a pressure P2 = 1 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)50.3 kJ
B)63.5 kJ
C)95.9 kJ
D)131 kJ
E)158 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
Use the following to answer the question: 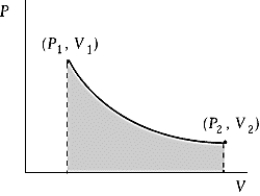
An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasi-static,isothermal expansion until its pressure is reduced to 50 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)416 J

An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasi-static,isothermal expansion until its pressure is reduced to 50 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)416 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
Use the following to answer the question: 
An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)The internal energy does not change during this process.

An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)The internal energy does not change during this process.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
The pressure of a gas in an isobaric expansion remains constant.In such an expansion,
A)no work is done.
B)work is done by the gas.
C)work is done on the gas.
D)"isobaric" and "expansion" are contradictory terms.
E)work is or is not done depending on whether the temperature of the gas changes.
A)no work is done.
B)work is done by the gas.
C)work is done on the gas.
D)"isobaric" and "expansion" are contradictory terms.
E)work is or is not done depending on whether the temperature of the gas changes.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
An ideal gas undergoes a cyclic process in which total (positive)work W is done by the gas.What total heat is added to the gas in one cycle?
A)W
B)-W
C)zero
D)2W
E)W/2
A)W
B)-W
C)zero
D)2W
E)W/2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
The equation of state for a certain gas under isothermal conditions is  where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
A)2.86 J
B)28.6 J
C)43.3 J
D)71.8 J
E)115 J
 where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximately
where the units are SI.The work done by this gas as its volume increases isothermally from 0.2 m3 to 0.8 m3 is approximatelyA)2.86 J
B)28.6 J
C)43.3 J
D)71.8 J
E)115 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
From the measured molar heat capacities and the equipartition theorem,for a polyatomic gas molecule the number of degrees of freedom due to translational motion are
A)3
B)6
C)5
D)2
E)7
A)3
B)6
C)5
D)2
E)7

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
Use the following to answer the question: 
An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)416 J

An ideal gas initially at 50ºC and pressure P1 = 100 kPa occupies a volume V1 = 3 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 50 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)208 J
C)256 J
D)304 J
E)416 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
Use the following to answer the question: 
An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)The internal energy does not change during this process.

An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much does the internal energy of the gas change during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)The internal energy does not change during this process.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Use the following to answer the question: 
-The internal energy for a diatomic gas is given by U = 5nRT/2.Calculate the internal energy of a 100 g mixture of oxygen (20%)and nitrogen (80%)gas at 25 C.(The molar weight of O2 = 32 g,and the molar weight of N2 = 28 g.)
A)21.6 kJ
B)1.80 kJ
C)12.1 kJ
D)13.0 kJ
E)1.10 kJ

-The internal energy for a diatomic gas is given by U = 5nRT/2.Calculate the internal energy of a 100 g mixture of oxygen (20%)and nitrogen (80%)gas at 25 C.(The molar weight of O2 = 32 g,and the molar weight of N2 = 28 g.)
A)21.6 kJ
B)1.80 kJ
C)12.1 kJ
D)13.0 kJ
E)1.10 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
Use the following to answer the question: 
An ideal gas initially at 50ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)850 J

An ideal gas initially at 50ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much work was done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)850 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
The equation of state for a certain gas under isothermal conditions is  where the units are SI.The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximately
where the units are SI.The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximately
A)13.6 J
B)31.2 J
C)71.8 J
D)281 J
E)312 J
 where the units are SI.The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximately
where the units are SI.The work done by this gas as its volume increases isothermally from 1 L to 10 L is approximatelyA)13.6 J
B)31.2 J
C)71.8 J
D)281 J
E)312 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Use the following to answer the question: 
-One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 6 atm and a temperature of 50ºC to a pressure P2 = 4 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)50.3 kJ
B)56.2 kJ
C)95.9 kJ
D)131 kJ
E)158 kJ

-One mole of an ideal gas ( = 5/3)expands adiabatically and quasistatically from a pressure P1 = 6 atm and a temperature of 50ºC to a pressure P2 = 4 atm.How much work is done by the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)50.3 kJ
B)56.2 kJ
C)95.9 kJ
D)131 kJ
E)158 kJ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
From the measured molar heat capacities and the equipartition theorem,for a diatomic gas molecule the number of degrees of freedom from rotational motion are
A)3
B)0
C)5
D)2
E)6
A)3
B)0
C)5
D)2
E)6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Use the following to answer the question: 
An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)850 J

An ideal gas initially at 100ºC and pressure P1 = 250 kPa occupies a volume V1 = 4.5 L.It undergoes a quasistatic,isothermal expansion until its pressure is reduced to 150 kPa.How much heat enters the gas during this process? R = 8.314 J/mol·K = 8.206 L·atm/mol·K.
A)116 J
B)320 J
C)575 J
D)640 J
E)850 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
An ideal gas is heated so that it expands at constant pressure.The gas does work W.What heat is added to the gas?
A)W
B)-W
C)zero
D)more than W
E)less than W
A)W
B)-W
C)zero
D)more than W
E)less than W

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
The work done by an ideal gas in an isothermal expansion from volume V1 to volume V2 is given by the formula:  Standard atmospheric pressure (1 atm)is 101.3 kPa.If 1.0 L of He gas at room temperature (20ºC)and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL,how much work is done on the gas?
Standard atmospheric pressure (1 atm)is 101.3 kPa.If 1.0 L of He gas at room temperature (20ºC)and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL,how much work is done on the gas?
A)5.6 kJ
B)4.7 102 J
C)4.7 102 kJ
D)2.3 102 kJ
E)2.3 102 J
 Standard atmospheric pressure (1 atm)is 101.3 kPa.If 1.0 L of He gas at room temperature (20ºC)and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL,how much work is done on the gas?
Standard atmospheric pressure (1 atm)is 101.3 kPa.If 1.0 L of He gas at room temperature (20ºC)and 1.0 atm of pressure is compressed isothermally to a volume of 100 mL,how much work is done on the gas?A)5.6 kJ
B)4.7 102 J
C)4.7 102 kJ
D)2.3 102 kJ
E)2.3 102 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A liquid is irregularly stirred in a well-insulated container and thereby undergoes a rise in temperature.Regarding the liquid as a system,you can say that
A)heat has been transferred.
B)the rise in temperature indicates work done by the system.
C)the internal energy has been unchanged.
D)the work done by the system equals the work done on the system.
E)there is a positive change in internal energy.
A)heat has been transferred.
B)the rise in temperature indicates work done by the system.
C)the internal energy has been unchanged.
D)the work done by the system equals the work done on the system.
E)there is a positive change in internal energy.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


