Deck 34: Wave Particle Duality and Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 34: Wave Particle Duality and Quantum Physics
1
In a photoelectric experiment,the threshold frequency for a material is 3.2 1014 Hz.An electron ejected from this surface by a photon of frequency 9.4 1014 Hz can be stopped by a stopping potential of
A)8.5 mV
B)1.6 V
C)0.26 nV
D)2.6 V
E)3.6 kV
A)8.5 mV
B)1.6 V
C)0.26 nV
D)2.6 V
E)3.6 kV
2.6 V
2
The maximum wavelength for the photoemission of electrons from a metal surface depends on
A)applied voltage.
B)light intensity.
C)the metal that the light strikes.
D)current.
E)None of these is correct.
A)applied voltage.
B)light intensity.
C)the metal that the light strikes.
D)current.
E)None of these is correct.
the metal that the light strikes.
3
Potassium has a work function of 2.3 eV for photoelectric emission.Which of the following wavelengths is the longest wavelength for which photoemission occurs?
A)400 nm
B)450 nm
C)500 nm
D)540 nm
E)600 nm
A)400 nm
B)450 nm
C)500 nm
D)540 nm
E)600 nm
500 nm
4
Which of the following statements concerning particles and waves is/are correct?
A)Uncharged particles travel in straight lines until they collide with something.
B)When two particles meet in space,they never produce an interference pattern.
C)When a large number of small particles exchange a small amount of energy,the exchange cannot be distinguished from that of a wave.
D)When two waves of equal intensity and originating from coherent sources meet in space,the resulting wave can have an intensity anywhere from zero to four times the intensity of each of the original waves.
E)All of the above are correct.
A)Uncharged particles travel in straight lines until they collide with something.
B)When two particles meet in space,they never produce an interference pattern.
C)When a large number of small particles exchange a small amount of energy,the exchange cannot be distinguished from that of a wave.
D)When two waves of equal intensity and originating from coherent sources meet in space,the resulting wave can have an intensity anywhere from zero to four times the intensity of each of the original waves.
E)All of the above are correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
Use the following figure for the next two questions. 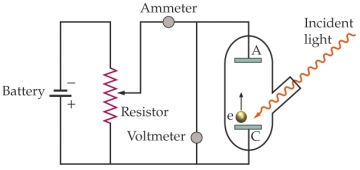
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.5 10-19 J.The battery is set at 1.5 V.What is the longest wavelength of light needed to produce an electric current from the cathode (C)to the anode (A)?
A)337 nm
B)354 nm
C)620 nm
D)827 nm
E)None of these is correct.

Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.5 10-19 J.The battery is set at 1.5 V.What is the longest wavelength of light needed to produce an electric current from the cathode (C)to the anode (A)?
A)337 nm
B)354 nm
C)620 nm
D)827 nm
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following experiment(s)illustrates the particle nature of light?
A)Young's double slit experiment
B)the photoelectric effect
C)Compton scattering
D)(A)and (B)
E)(B)and (C)
A)Young's double slit experiment
B)the photoelectric effect
C)Compton scattering
D)(A)and (B)
E)(B)and (C)

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
Light of wavelength 400 nm is incident on a certain metal.The stopping potential for the emitted electrons is measured to be 1.2 V.What is the work function of this metal? (Planck's constant h = 6.626 10-34 J · s = 4.136 10-15 eV s.)
A)4.3 eV
B)3.1 eV
C)1.9 eV
D)1.2 eV
E)0.95 eV
A)4.3 eV
B)3.1 eV
C)1.9 eV
D)1.2 eV
E)0.95 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about Maxwell's theory of electromagnetism is false?
A)The theory shows that light travels as transverse waves.
B)The theory shows that the speed of light is c in vacuum.
C)The theory shows that light travels as photons.
D)The theory shows that light consists of electric and magnetic waves.
E)The theory shows that light does not depend on a medium for it to travel.
A)The theory shows that light travels as transverse waves.
B)The theory shows that the speed of light is c in vacuum.
C)The theory shows that light travels as photons.
D)The theory shows that light consists of electric and magnetic waves.
E)The theory shows that light does not depend on a medium for it to travel.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
The velocity of escape of photoelectrons
A)increases with increasing frequency of the incident light.
B)decreases with increasing frequency of the incident light.
C)is independent of the frequency of the incident light.
D)is directly proportional to the intensity of the incident light.
E)depends only on the intensity of the incident light.
A)increases with increasing frequency of the incident light.
B)decreases with increasing frequency of the incident light.
C)is independent of the frequency of the incident light.
D)is directly proportional to the intensity of the incident light.
E)depends only on the intensity of the incident light.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
If the frequency of light causing photoemission of electrons is doubled,the kinetic energy of the ejected electrons
A)increases by a factor of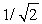
B)doubles.
C)decreases by a factor of 2.
D)increases by a factor less than two.
E)increases by a factor more than two.
A)increases by a factor of
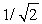
B)doubles.
C)decreases by a factor of 2.
D)increases by a factor less than two.
E)increases by a factor more than two.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
The wavelength of blue light is closest to
A)400 nm
B)500 nm
C)600 nm
D)700 nm
E)800 nm
A)400 nm
B)500 nm
C)600 nm
D)700 nm
E)800 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
Estimate the number of photons emitted by the Sun in a second.The power output from the Sun is 4 1026 W and assume that the average wavelength of each photon is 550 nm.
A)1026
B)1045
C)1023
D)1028
E)none of the above
A)1026
B)1045
C)1023
D)1028
E)none of the above

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
The visible portion of the electromagnetic spectrum extends from
A)200 nm to 500 nm.
B)300 nm to 600 nm.
C)400 nm to 700 nm.
D)500 nm to 800 nm.
E)600 nm to 900 nm.
A)200 nm to 500 nm.
B)300 nm to 600 nm.
C)400 nm to 700 nm.
D)500 nm to 800 nm.
E)600 nm to 900 nm.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
Photons with an energy of 7.52 eV strike a material that has a work function of 4.22 eV.The maximum kinetic energy of the electron emitted from this material is
A)7.5 eV
B)12 eV
C)3.3 eV
D)0.98 eV
E)No electrons are ejected by these photons.
A)7.5 eV
B)12 eV
C)3.3 eV
D)0.98 eV
E)No electrons are ejected by these photons.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
The maximum kinetic energy of electrons ejected from barium (whose work function is 2.50 eV)when it is illuminated by light of wavelength 350 nm is
A)0.20 eV
B)0.41 eV
C)0.63 eV
D)0.95 eV
E)1.05 eV
A)0.20 eV
B)0.41 eV
C)0.63 eV
D)0.95 eV
E)1.05 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
Albert Einstein was awarded the Nobel Prize for his
A)theory of special relativity.
B)theory of general relativity.
C)explanation of Brownian motion.
D)explanation of the Michelson-Morley experiment.
E)explanation of the photoelectric effect.
A)theory of special relativity.
B)theory of general relativity.
C)explanation of Brownian motion.
D)explanation of the Michelson-Morley experiment.
E)explanation of the photoelectric effect.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
In Young's experiment,
A)each slit acts as a line source.
B)an interference pattern is observed on a screen placed behind the slits.
C)interference maxima occur at angles such that the path difference is an integral number of wavelengths.
D)interference minima occur when the path difference is one half wavelength or any odd number of half wavelengths.
E)all of the above are correct.
A)each slit acts as a line source.
B)an interference pattern is observed on a screen placed behind the slits.
C)interference maxima occur at angles such that the path difference is an integral number of wavelengths.
D)interference minima occur when the path difference is one half wavelength or any odd number of half wavelengths.
E)all of the above are correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
The wavelength of red light is closest to
A)100 nm
B)300 nm
C)500 nm
D)700 nm
E)900 nm
A)100 nm
B)300 nm
C)500 nm
D)700 nm
E)900 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
The work function for tungsten is 4.58 eV.What is the kinetic energy of electrons emitted when light of wavelength 400 nm is incident on a tungsten surface? (Planck's constant h = 6.626 10-34 J · s = 4.136 10-15 eV · s.)
A)0.74 eV
B)1.5 eV
C)7.7 eV
D)2.9 eV
E)no electrons are emitted
A)0.74 eV
B)1.5 eV
C)7.7 eV
D)2.9 eV
E)no electrons are emitted

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
In the photoelectric effect,the work function depends on the
A)incident wavelength.
B)applied voltage.
C)light intensity.
D)metal that the light strikes.
E)current.
A)incident wavelength.
B)applied voltage.
C)light intensity.
D)metal that the light strikes.
E)current.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the photon energy for light of wavelength 500 nm.(Planck's constant h = 6.626 10-34 J·s)
A)1.24 eV
B)1.98 eV
C)2.24 eV
D)2.48 eV
E)2.98 eV
A)1.24 eV
B)1.98 eV
C)2.24 eV
D)2.48 eV
E)2.98 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
A Compton-scattered X-ray photon has less energy than the incident photon.The scattered photon therefore
A)has the same wavelength as the incident photon.
B)has a longer wavelength than the incident photon.
C)has a shorter wavelength than the incident photon.
D)cannot be assigned a wavelength.
E)has none of these wavelengths.
A)has the same wavelength as the incident photon.
B)has a longer wavelength than the incident photon.
C)has a shorter wavelength than the incident photon.
D)cannot be assigned a wavelength.
E)has none of these wavelengths.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons.What is the energy of the scattered photons whose angle of scattering is 45º?
A)13.3 10-14 J
B)9.00 10-14 J
C)28.0 10-14 J
D)18.0 10-14 J
E)2.14 10-14 J
A)13.3 10-14 J
B)9.00 10-14 J
C)28.0 10-14 J
D)18.0 10-14 J
E)2.14 10-14 J

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
Light falling on the surface of a metal such as cesium can liberate electrons from the metal.The kinetic energy of electrons emitted from a metal can be increased by
A)using light of higher frequency.
B)using light of lower frequency.
C)increasing the intensity of the incident light.
D)using a metal with a greater work function.
E)None of these is correct.
A)using light of higher frequency.
B)using light of lower frequency.
C)increasing the intensity of the incident light.
D)using a metal with a greater work function.
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
The man whose name is most closely associated with the explanation of the photoelectric effect is
A)M.Planck.
B)E.Fermi.
C)N.Bohr.
D)E.Rutherford.
E)A.Einstein.
A)M.Planck.
B)E.Fermi.
C)N.Bohr.
D)E.Rutherford.
E)A.Einstein.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
The maximum kinetic energy of photoelectrons produced in the photoelectric effect depends directly on the
A)frequency of the incident photons.
B)intensity of the incident photons.
C)area of the metal surface from which the photoelectrons are released.
D)thickness of the metal.
E)photoelectric current.
A)frequency of the incident photons.
B)intensity of the incident photons.
C)area of the metal surface from which the photoelectrons are released.
D)thickness of the metal.
E)photoelectric current.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
In the Compton effect,
A)an electron is stopped suddenly.
B)an X ray collides with an electron,the electron is ejected,and the X-ray photon ceases to exist.
C)a neutrino is produced in a nuclear decay.
D)an energetic photon collides with an electron,and both the electron and the photon are scattered.
E)None of these is correct.
A)an electron is stopped suddenly.
B)an X ray collides with an electron,the electron is ejected,and the X-ray photon ceases to exist.
C)a neutrino is produced in a nuclear decay.
D)an energetic photon collides with an electron,and both the electron and the photon are scattered.
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
Photons of wavelength 0.00150 nm undergo Compton collisions with free electrons.What is the energy of the scattered photons whose angle of scattering is 60º?
A)13.3 10-14 J
B)3.27 10-14 J
C)28.0 10-14 J
D)7.36 10-14 J
E)2.14 10-14 J
A)13.3 10-14 J
B)3.27 10-14 J
C)28.0 10-14 J
D)7.36 10-14 J
E)2.14 10-14 J

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following formulas has the correct units for the linear momentum of a photon?
A)hc/
B)c/n
C)l/
D) /h
E)None of these is correct.
A)hc/
B)c/n
C)l/
D) /h
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
What is the momentum (in SI units)of a photon of wavelength = 410 nm? (Planck's constant h = 6.626 10-34 J·s)
A)1.62 10-27 kg m/s
B)2.45 10-27 kg m/s
C)3.23 10-27 kg m/s
D)4.67 10-27 kg m/s
E)5.29 10-27 kg m/s
A)1.62 10-27 kg m/s
B)2.45 10-27 kg m/s
C)3.23 10-27 kg m/s
D)4.67 10-27 kg m/s
E)5.29 10-27 kg m/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
What is the shift in wavelength of a photon scattered off an electron at 160º?
A)2.4 pm
B)0.15 pm
C)1.6 pm
D)1.2 pm
E)4.7 pm
A)2.4 pm
B)0.15 pm
C)1.6 pm
D)1.2 pm
E)4.7 pm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the photon energy for light of wavelength 600 nm.(Planck's constant h = 6.626 10-34 J·s)
A)1.24 eV
B)1.98 eV
C)2.07 eV
D)2.48 eV
E)2.98 eV
A)1.24 eV
B)1.98 eV
C)2.07 eV
D)2.48 eV
E)2.98 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
An X-ray photon scattering off an electron imparts some of its energy to the electron (the Compton effect).Which of the following statements is true?
A)The wavelength of the scattered photon is unchanged.
B)The wavelength of the scattered photon is decreased.
C)The wavelength of the scattered photon is increased.
D)The scattered photon gains speed.
E)The scattered photon is slowed.
A)The wavelength of the scattered photon is unchanged.
B)The wavelength of the scattered photon is decreased.
C)The wavelength of the scattered photon is increased.
D)The scattered photon gains speed.
E)The scattered photon is slowed.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
When a certain X ray is Compton scattered at right angles to its initial direction,the shift in its wavelength is c = 2.4 pm (1 picometer = 10-12 m).If the wavelength of this X ray is 15.4 pm,the wavelength of the scattered X ray must be closest to
A)2.4 pm
B)0.15 pm
C)18 pm
D)13 pm
E)170 pm
A)2.4 pm
B)0.15 pm
C)18 pm
D)13 pm
E)170 pm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
The photoelectric threshold of a certain metal is 310 nm.The maximum kinetic energy of electrons ejected from the surface of the metal by ultraviolet light of wavelength 200 nm is
A)2.22 eV
B)2.60 eV
C)10.2 eV
D)12.3 eV
E)none of these because no electrons are ejected
A)2.22 eV
B)2.60 eV
C)10.2 eV
D)12.3 eV
E)none of these because no electrons are ejected

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
36
Use the following figure for the next two questions. 
Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.8 10-19 J.Light of a wavelength 350 nm is incident on the material.What is the lowest voltage needed between the cathode (C)and the anode (A)to stop any electrons ejected from the cathode from reaching the anode?
A)2.37 V
B)1.17 V
C)3.54 V
D)4.71 V
E)None of these is correct.

Apparatus for studying the photoelectric effect
-The work function of the material being investigated is 3.8 10-19 J.Light of a wavelength 350 nm is incident on the material.What is the lowest voltage needed between the cathode (C)and the anode (A)to stop any electrons ejected from the cathode from reaching the anode?
A)2.37 V
B)1.17 V
C)3.54 V
D)4.71 V
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
37
What is the momentum (in SI units)of a photon of wavelength = 560 nm? (Planck's constant h = 6.626 10-34 J·s.)
A)3.7 10-42 kg · m/s
B)8.4 10-25 kg · m/s
C)1.2 10-30 kg · m/s
D)1.2 10-27 kg · m/s
E)3.6 10-27 kg · m/s
A)3.7 10-42 kg · m/s
B)8.4 10-25 kg · m/s
C)1.2 10-30 kg · m/s
D)1.2 10-27 kg · m/s
E)3.6 10-27 kg · m/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
If the work function of thoriated tungsten is 4 10-19 J,the longest wavelength of light that will cause photoelectrons to be emitted is approximately
A)880 nm
B)400 nm
C)495 nm
D)700 nm
E)181 nm
A)880 nm
B)400 nm
C)495 nm
D)700 nm
E)181 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
Photon A has twice the energy of photon B.The ratio of the momentum of A to that of B is
A)4
B)2
C)1
D)1/2
E)1/4
A)4
B)2
C)1
D)1/2
E)1/4

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
What is the momentum (in SI units)of a photon of wavelength = 480 nm? (Planck's constant h = 6.626 10-34 J·s)
A)7.24 10-27 kg m/s
B)6.21 10-27 kg m/s
C)4.14 10-27 kg m/s
D)2.76 10-27 kg m/s
E)1.38 10-27 kg m/s
A)7.24 10-27 kg m/s
B)6.21 10-27 kg m/s
C)4.14 10-27 kg m/s
D)2.76 10-27 kg m/s
E)1.38 10-27 kg m/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
An electron in the hydrogen atom makes a transition from an energy level of -1.51 eV to one of energy -3.40 eV.In this process a photon is emitted with what wavelength?
A)1.89 nm
B)656 nm
C)456 nm
D)821 nm
E)365 nm
A)1.89 nm
B)656 nm
C)456 nm
D)821 nm
E)365 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction.It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon.Calculate the difference in energy between the first and second recoiling electrons.
A)606 keV
B)194 keV
C)488 keV
D)118 keV
E)370 keV
A)606 keV
B)194 keV
C)488 keV
D)118 keV
E)370 keV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
For an electron to have a de Broglie wavelength of 0.10 nm it must be accelerated through a potential difference of
A)1.0 V
B)0.51 MV
C)0.15 kV
D)5 kV
E)0.94 kV
A)1.0 V
B)0.51 MV
C)0.15 kV
D)5 kV
E)0.94 kV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
When a surface is illuminated with light of wavelength 500 nm,the maximum speed of the photoelectrons is 300 km/s.When the same surface is illuminated by light of half this wavelength what is the maximum speed of the photoelectrons in this case?
A)680 km/s
B)1280 km/s
C)885 km/s
D)980 km/s
E)14800 km/s
A)680 km/s
B)1280 km/s
C)885 km/s
D)980 km/s
E)14800 km/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
A gamma-ray photon of energy 1.00 MeV scatters off an electron at an angle perpendicular to its original direction.Calculate the kinetic energy of the recoiling electron.
A)489 keV
B)511 keV
C)663 keV
D)253 keV
E)337 keV
A)489 keV
B)511 keV
C)663 keV
D)253 keV
E)337 keV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
A 10.0-kg mass moving with a velocity of 100 m/s has a de Broglie wavelength of approximately
A)1.98 10-22 m
B)1.51 1036 m
C)6.62 10-33 m
D)6.62 10-37 m
E)6.62 10-31 m
A)1.98 10-22 m
B)1.51 1036 m
C)6.62 10-33 m
D)6.62 10-37 m
E)6.62 10-31 m

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
In Compton scattering of X rays by electrons,the maximum shift in the X-ray wavelength occurs when the scattering angle is
A)180º
B)135º
C)90º
D)45º
E)0º
A)180º
B)135º
C)90º
D)45º
E)0º

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
A gamma-ray photon is back scattered at an angle of 180° after a collision with an electron.If the scattered photon has energy 150 keV,what was the energy of the initial photon?
A)303 keV
B)363 keV
C)95 keV
D)427 keV
E)393 keV
A)303 keV
B)363 keV
C)95 keV
D)427 keV
E)393 keV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
An X-ray photon of wavelength 0.10 nm is Compton-scattered from a free electron.The greatest observed shift in wavelength,as seen in the scattered photon,is
A)0.0012 nm
B)0.0024 nm
C)0.0048 nm
D)0.18 nm
E)0.10 nm
A)0.0012 nm
B)0.0024 nm
C)0.0048 nm
D)0.18 nm
E)0.10 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
A gamma-ray photon of energy 800 keV scatters off an electron at an angle perpendicular to its original direction.It then scatters off a second electron such that this secondary scattered photon continues in the direction of the original photon.Calculate the energy difference between the initial and final photon.
A)682 keV
B)194 keV
C)606 keV
D)118 keV
E)312 keV
A)682 keV
B)194 keV
C)606 keV
D)118 keV
E)312 keV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
The pupil area of the human eye is ~ 1 10-5 m2.The minimum light intensity that the eye is sensitive to is ~ 1 10-10 Wm-2.How many photons per second of wavelength 550 nm does this correspond to?
A)~300 s-1
B)~30000 s-1
C)~3000 s-1
D)~3 10-2 s-1
E)~3 1012 s-1
A)~300 s-1
B)~30000 s-1
C)~3000 s-1
D)~3 10-2 s-1
E)~3 1012 s-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
The dissociation energy is the energy required to separate the two atoms in a diatomic molecule in their ground-state.If the dissociation energy of molecular oxygen is 7.2 eV,then calculate the maximum wavelength of light from the Sun that can break apart atmospheric O2.
A)1.7 10-7 nm
B)1.7 10-8 m
C)1.7 10-9 m
D)1.7 10-7 m
E)1.7 102 m
A)1.7 10-7 nm
B)1.7 10-8 m
C)1.7 10-9 m
D)1.7 10-7 m
E)1.7 102 m

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
The Davisson and Germer experiment verified that
A)the velocity of light in a vacuum is a universal constant.
B)electrons have an intrinsic spin.
C)waves have a particle aspect.
D)electrons show wave characteristics.
E)the neutron does not have a charge.
A)the velocity of light in a vacuum is a universal constant.
B)electrons have an intrinsic spin.
C)waves have a particle aspect.
D)electrons show wave characteristics.
E)the neutron does not have a charge.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
Microwaves range in wavelength from about 2.0 10-2 cm to about 5.0 cm.What is the maximum energy in eV that a microwave may have?
A)0.025 meV
B)6.2 meV
C)2.5 eV
D)6.2 eV
E)0.62 meV
A)0.025 meV
B)6.2 meV
C)2.5 eV
D)6.2 eV
E)0.62 meV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
An electron (me = 9.11 10-31 kg)traveling with a velocity of 5.50 106 m/s has a de Broglie wavelength of approximately
A)0.0710 nm
B)0.130 nm
C)0.180 nm
D)0.230 nm
E)0.550 nm
A)0.0710 nm
B)0.130 nm
C)0.180 nm
D)0.230 nm
E)0.550 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
An electron (me = 9.11 10-31 kg)traveling with a velocity of 1.1 106 m/s has a de Broglie wavelength of approximately
A)0.66 nm
B)0.78 nm
C)0.89 nm
D)0.97 nm
E)1.2 nm
A)0.66 nm
B)0.78 nm
C)0.89 nm
D)0.97 nm
E)1.2 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
Magnetic Resonance Imaging (MRI)is a much used noninvasive diagnostic technique in medicine.The radio frequency photons used have energies of about 10-7 eV.What wavelength does this correspond to?
A)12 nm
B)1.2 m
C)12 m
D)1.2 nm
E)120 m
A)12 nm
B)1.2 m
C)12 m
D)1.2 nm
E)120 m

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
The work function for a metal is 5.58 eV.What is the threshold wavelength for the photoelectric effect of incident photons?
A)2.22 10-7 m
B)2.22 10-9 m
C)4.50 106 m
D)4.50 10-6 m
E)2.22 10-11 m
A)2.22 10-7 m
B)2.22 10-9 m
C)4.50 106 m
D)4.50 10-6 m
E)2.22 10-11 m

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
The work functions for two different metals A and B,are 3.23 eV and 5.33 eV,respectively.If photons of 220 nm are incident on the two metals,calculate the difference in energy of the fastest photoelectrons from metal A and metal B.
A)0.69 eV
B)0.0 eV
C)2.10 eV
D)1.72 eV
E)2.41 eV
A)0.69 eV
B)0.0 eV
C)2.10 eV
D)1.72 eV
E)2.41 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
In a Compton scattering experiment,an 8.00-MeV incident photon is scattered at an angle of 15º with energy of 5.20 MeV.The kinetic energy of the recoil electron is
A)2.80 MeV
B)2.08 MeV
C)5.20 MeV
D)7.28 MeV
E)800 MeV
A)2.80 MeV
B)2.08 MeV
C)5.20 MeV
D)7.28 MeV
E)800 MeV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
The speed of an electron whose de Broglie wavelength is 0.0010 m is approximately
A)7.3 10-7 m/s
B)7.3 10-4 m/s
C)0.73 m/s
D)0.51 km/s
E)5.1 105 m/s
A)7.3 10-7 m/s
B)7.3 10-4 m/s
C)0.73 m/s
D)0.51 km/s
E)5.1 105 m/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
The electron microscope is a welcome addition to the field of microscopy because electrons have a __________ wavelength than light,thereby increasing the __________ of the microscope.
A)longer; resolving power
B)longer; breadth of field
C)shorter; resolving power
D)longer; intensity
E)None of these is correct.
A)longer; resolving power
B)longer; breadth of field
C)shorter; resolving power
D)longer; intensity
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
If a baseball,an electron,and a photon all have the same momentum,which has the longest wavelength?
A)baseball
B)electron
C)photon
D)all have the same wavelength
E)it depends on the energy of the photon
A)baseball
B)electron
C)photon
D)all have the same wavelength
E)it depends on the energy of the photon

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the wavelength of light of energy 1 keV divided by the de Broglie wavelength of a proton of kinetic energy 1 meV.
A)1.4
B)1.0 10-6
C)1.0 106
D)1.9
E)0.73
A)1.4
B)1.0 10-6
C)1.0 106
D)1.9
E)0.73

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
The momentum of a 20-MeV electron is approximately
A)1.6 10-21 kg · m/s
B)2.4 10-42 kg · m/s
C)5.8 10-42 kg · m/s
D)2.4 10-21 kg · m/s
E)5.8 10-21 kg · m/s
A)1.6 10-21 kg · m/s
B)2.4 10-42 kg · m/s
C)5.8 10-42 kg · m/s
D)2.4 10-21 kg · m/s
E)5.8 10-21 kg · m/s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following particles has the longest wavelength?
A)a photon with a frequency of 3 1019 Hz
B)a photon with an energy of 2 10-13 J
C)an electron with a momentum of 6.6 10-21 kg · m/s
D)a proton with a momentum of 6.6 10-21 kg · m/s
E)all four have the same wavelength
A)a photon with a frequency of 3 1019 Hz
B)a photon with an energy of 2 10-13 J
C)an electron with a momentum of 6.6 10-21 kg · m/s
D)a proton with a momentum of 6.6 10-21 kg · m/s
E)all four have the same wavelength

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
A proton has five times the momentum of an electron.If the electron has a de Broglie wavelength ,then the de Broglie wavelength of the proton is
A)
B)5
C) /5
D)25
E) /25
A)
B)5
C) /5
D)25
E) /25

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
The wave-particle duality theory is the first adequate explanation of which one of the following observations about the hydrogen atom?
A)The proton attracts the electron.
B)The proton forms the nucleus of the atom.
C)The electron orbits the proton.
D)The mass of the atom is less than the sum of the individual proton and electron masses.
E)The ground-state atom is stable to radiative collapse.
A)The proton attracts the electron.
B)The proton forms the nucleus of the atom.
C)The electron orbits the proton.
D)The mass of the atom is less than the sum of the individual proton and electron masses.
E)The ground-state atom is stable to radiative collapse.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
The wave function for sound waves is
A)either the displacement of air molecules s,or the density .
B)the wave function .
C)the electric field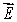 .
.
D)the string displacement y.
E)None of these is correct.
A)either the displacement of air molecules s,or the density .
B)the wave function .
C)the electric field
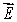 .
.D)the string displacement y.
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
Use the following figure for the next problem. 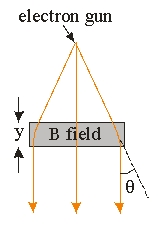
-An electron that is not localized in space is described by the wave function = A sin (kx - t).The kinetic energy of the electron is 10 keV.The value of is approximately
A)1.5 1019 s-1
B)3.0 1019 s-1
C)4.5 1019 s-1
D)1.5 1021 s-1
E)3.0 1021 s-1

-An electron that is not localized in space is described by the wave function = A sin (kx - t).The kinetic energy of the electron is 10 keV.The value of is approximately
A)1.5 1019 s-1
B)3.0 1019 s-1
C)4.5 1019 s-1
D)1.5 1021 s-1
E)3.0 1021 s-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
Electrons do not exhibit wave properties as readily as light because electrons typically have much __________ momenta than light and hence much __________ wavelengths.
A)greater; longer
B)greater; shorter
C)lesser; longer
D)lesser; shorter
E)greater; the same
A)greater; longer
B)greater; shorter
C)lesser; longer
D)lesser; shorter
E)greater; the same

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
An alpha particle (mass = 4 amu)is moving twice as fast as a proton (mass = 1 amu).Calculate the de Broglie wavelength of the proton divided by the de Broglie wavelength of the alpha particle.
A)1/4
B)8
C)1/8
D)2
E)1/2
A)1/4
B)8
C)1/8
D)2
E)1/2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
Use the following figure for the next problem. 
The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV.The wavelength of the electrons is
A)2.14 nm
B)3.88 pm
C)4.87 nm
D)12.4 pm
E)6.87 nm

The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV.The wavelength of the electrons is
A)2.14 nm
B)3.88 pm
C)4.87 nm
D)12.4 pm
E)6.87 nm

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
Use the following figure for the next problem. 
-An electron that is not localized in space is described by the wave function = A sin(kx - t).The kinetic energy of the electron is 10 keV.The value of k is approximately
A)1.6 10-11 m-1
B)3.2 1013 m-1
C)1.6 1011 m-1
D)3.2 1011 m-1
E)5.1 1011 m-1

-An electron that is not localized in space is described by the wave function = A sin(kx - t).The kinetic energy of the electron is 10 keV.The value of k is approximately
A)1.6 10-11 m-1
B)3.2 1013 m-1
C)1.6 1011 m-1
D)3.2 1011 m-1
E)5.1 1011 m-1

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
The fact that particles have a wave nature was first verified by
A)the Heisenberg uncertainty principle.
B)Davisson and Germer's diffraction of electrons experiment.
C)blackbody radiation studies.
D)the Bohr theory of the atom.
E)the photoelectric effect.
A)the Heisenberg uncertainty principle.
B)Davisson and Germer's diffraction of electrons experiment.
C)blackbody radiation studies.
D)the Bohr theory of the atom.
E)the photoelectric effect.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
The wave-particle duality theory is the first adequate explanation of which one of the following observations about the hydrogen atom?
A)More than one possible orbit exists for the electron.
B)An infinite number of possible orbits exists for the electron.
C)Only certain energies are possible for the orbiting electron.
D)More than one momentum is possible for the orbiting electron.
E)None of these is correct.
A)More than one possible orbit exists for the electron.
B)An infinite number of possible orbits exists for the electron.
C)Only certain energies are possible for the orbiting electron.
D)More than one momentum is possible for the orbiting electron.
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
A gamma-ray photon of energy 100 keV scatters off an electron at an angle perpendicular to its original direction.Calculate the de Broglie wavelength of the recoiling electron.
A)13.5 10-12 m
B)12.4 10-12 m
C)14.8 10-12 m
D)2.4 10-12 m
E)9.54 10-12 m
A)13.5 10-12 m
B)12.4 10-12 m
C)14.8 10-12 m
D)2.4 10-12 m
E)9.54 10-12 m

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
The wavelength of an electron is 1.33 nm.Its kinetic energy is approximately
A)1.18 eV
B)1.08 eV
C)0.922 eV
D)0.850 eV
E)3.40 eV
A)1.18 eV
B)1.08 eV
C)0.922 eV
D)0.850 eV
E)3.40 eV

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
Use the following figure for the next problem. 
-The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV.The electrons then pass through a region of magnetic field of distance y = 8 cm which points perpendicularly to the direction of travel of the electron.What is the strength of the magnetic field needed to deflect the electron by an angle of = 25 ? (Ignore any relativistic effect.1 Gauss = 10-4 T)
A)120.1 Gauss
B)30.4 Gauss
C)21.8 Gauss
D)14.6 Gauss
E)None of these is correct.

-The electrons coming out of the electron gun in an electron microscope are being accelerated by a potential of 100 kV.The electrons then pass through a region of magnetic field of distance y = 8 cm which points perpendicularly to the direction of travel of the electron.What is the strength of the magnetic field needed to deflect the electron by an angle of = 25 ? (Ignore any relativistic effect.1 Gauss = 10-4 T)
A)120.1 Gauss
B)30.4 Gauss
C)21.8 Gauss
D)14.6 Gauss
E)None of these is correct.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
Suppose that the world could be changed so that the de Broglie wavelength of a 40-kg runner who goes 100 m in 10.0 s was 5 m.Assuming that all other constants remain the same,the value of Planck's constant would have to be
A)0.80 J · s
B)1.3 J · s
C)0.76 J · s
D)2.0 kJ · s
E)0.20 MJ · s
A)0.80 J · s
B)1.3 J · s
C)0.76 J · s
D)2.0 kJ · s
E)0.20 MJ · s

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


