Deck 13: Conjugated Unsaturated Systems
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/201
Play
Full screen (f)
Deck 13: Conjugated Unsaturated Systems
1
The allyl radical has how many electrons in non-bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5
1
2
The allyl cation has how many bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5
1
3
The LUMO of 1,3-pentadiene has how many electrons in its ground state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0
0
4
The HOMO of 1,3-pentadiene has how many electrons in its ground state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
5
The allyl radical has how many electrons in bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
6
The allyl cation has how many molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
7
The LUMO of 1,3-pentadiene has how many electrons in its excited state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
8
1,3-Butadiene has how many electrons in its ground state bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
9
1,3-Butadiene has how many electrons in its ground state antibonding molecular orbitals?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
10
Which carbocation would be least stable? 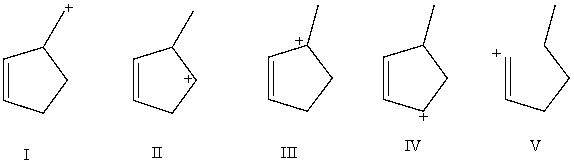
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
11
The HOMO of 1,3-pentadiene has how many electrons in its excited state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
12
The HOMO of the allylic cation has how many electrons in its ground state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
13
The allyl radical has how many bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
14
1,3-Pentadiene has how many bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
15
The LUMO of the allylic radical has how many electrons in its ground state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
16
The HOMO of the allylic radical has how many electrons in its ground state?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
17
The allyl cation has how many electrons in bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
18
The allyl radical has how many molecular orbitals?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
19
1,3-Pentadiene has how many antibonding molecular orbitals?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
20
1,3-Butadiene has how many bonding molecular orbitals?
A)1
B)2
C)3
D)4
E)0
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
21
Considering both configurational and conformational factors,select the least stable form of 2,4-hexadiene. 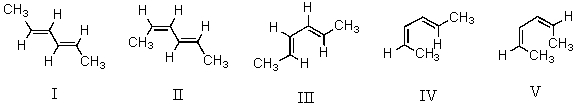
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
22
Which carbocation would be least stable? 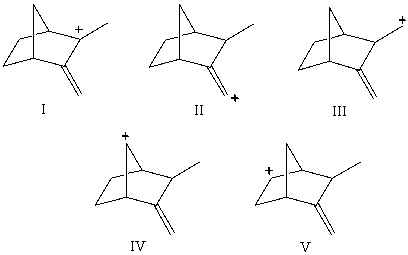
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
23
Arrange these carbocations in order of expected increasing stability. 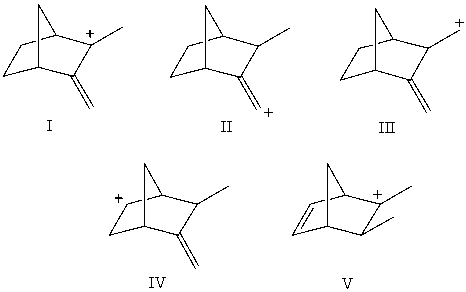
A)V < II < I < IV < III
B)V < I < II < IV < III
C)IV D)IV < III < I < II < V
E)II < III < IV < V < I

A)V < II < I < IV < III
B)V < I < II < IV < III
C)IV D)IV < III < I < II < V
E)II < III < IV < V < I

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following dienes would you expect to be the most stable?
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH=C(CH3)CH=CHCH2CH3
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH=C(CH3)CH=CHCH2CH3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
25
Which diene would be least stable? 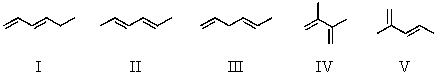
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
26
Which carbocation would be most stable? 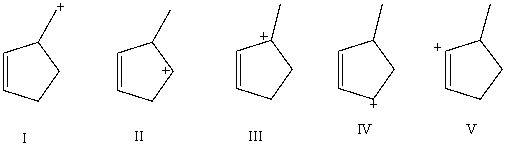
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
27
Arrange these hexadienes in order of expected increasing stability. 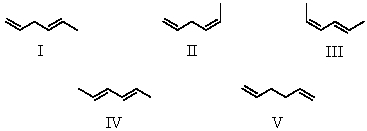
A)V < II < I < III < IV
B)III < IV < II < I < V
C)IV < III < II < V < I
D)IV < III < I < II < V
E)I < II < IV < III < V

A)V < II < I < III < IV
B)III < IV < II < I < V
C)IV < III < II < V < I
D)IV < III < I < II < V
E)I < II < IV < III < V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
28
Which alkene would you expect to be most stable?
A)CH2=CHCH2CH2CH=CH2
B)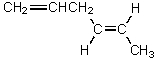
C)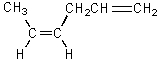
D)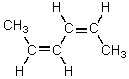
E)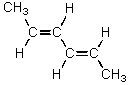
A)CH2=CHCH2CH2CH=CH2
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following dienes would you expect to be the most stable?
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH2CH=C=CHCH2CH3
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH2CH=C=CHCH2CH3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following dienes would you expect to be the most stable?
A)CH3CH=CHCH=CHCH3
B)CH3CH=CHCH2CH=CH2
C)CH2=CHCH2CH2CH=CH2
D)CH2=CHCH(CH3)CH=CH2
E)CH3CH=C=CHCH2CH3
A)CH3CH=CHCH=CHCH3
B)CH3CH=CHCH2CH=CH2
C)CH2=CHCH2CH2CH=CH2
D)CH2=CHCH(CH3)CH=CH2
E)CH3CH=C=CHCH2CH3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
31
Arrange these hexadienes in order of expected decreasing stability. 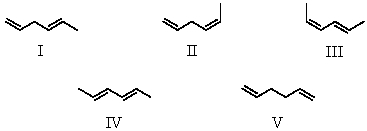
A)V > II > I > III > IV
B)III > IV > II > I > V
C)IV > III > II > V > I
D)IV > III > I > II > V
E)I > II > IV > III > V

A)V > II > I > III > IV
B)III > IV > II > I > V
C)IV > III > II > V > I
D)IV > III > I > II > V
E)I > II > IV > III > V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following dienes would you expect to be the least stable?
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH=C(CH3)CH=CHCH2CH3
A)CH3CH2CH=CHCH2CH=CHCH3
B)CH3CH=CHCH=CHCH2CH3
C)CH2=CHCH2CH2CH2CH=CH2
D)CH2=CHCH=CHCH2CH2CH3
E)CH3CH=C(CH3)CH=CHCH2CH3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds would be the most stable? 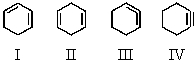
A)I
B)II
C)III
D)IV
E)They are all of equal stability.

A)I
B)II
C)III
D)IV
E)They are all of equal stability.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
34
Arrange these carbocations in order of expected increasing stability. 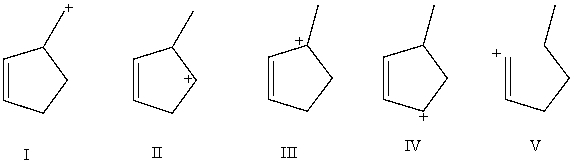
A)V < II < I < IV < III
B)V < I < II < IV < III
C)IV < I < II < V < III
D)IV < III < I < II < V
E)I < II < IV < III < V

A)V < II < I < IV < III
B)V < I < II < IV < III
C)IV < I < II < V < III
D)IV < III < I < II < V
E)I < II < IV < III < V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
35
Which carbocation would be most stable? 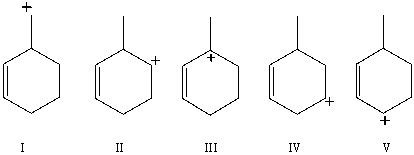
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
36
Considering both configurational and conformational factors,select the most stable form of 2,4-hexadiene. 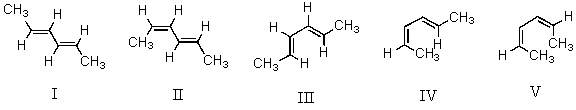
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
37
Which carbocation would be most stable?
A)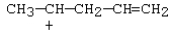
B)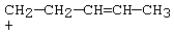
C)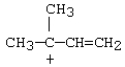
D)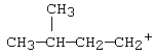
E)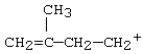
A)
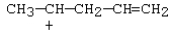
B)
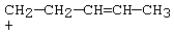
C)

D)
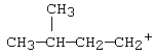
E)
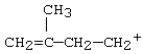

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
38
Which carbocation would be least stable?
A)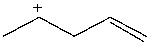
B)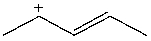
C)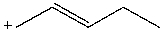
D)
E)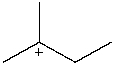
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
39
Which is not an example of resonance? 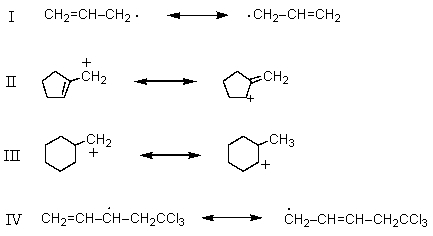
A)I
B)II
C)III
D)IV
E)None of these choices are examples of resonance.

A)I
B)II
C)III
D)IV
E)None of these choices are examples of resonance.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
40
Which carbocation would be most stable? 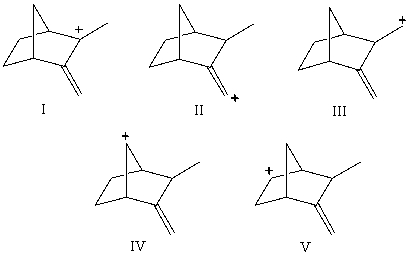
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following dienes is a cumulated diene?
A)CH2=CHCH2CH2CH2CH=CH2
B)CH2=CHCH=CHCH2CH2CH3
C)CH3CH=C=CHCH2CH2CH3
D)CH3CH=CHCH=CHCH2CH3
E)CH3CH2CH=CHCH2CH=CH2
A)CH2=CHCH2CH2CH2CH=CH2
B)CH2=CHCH=CHCH2CH2CH3
C)CH3CH=C=CHCH2CH2CH3
D)CH3CH=CHCH=CHCH2CH3
E)CH3CH2CH=CHCH2CH=CH2

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
42
Which pair does not represent a pair of resonance structures? 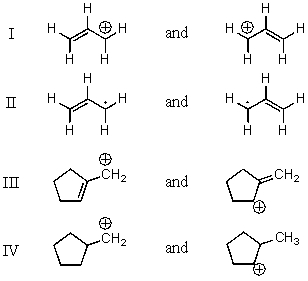
A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
43
Select the structure of the conjugated diene. 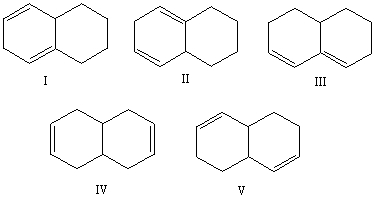
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
44
The substituent R on the bicyclic compound shown is considered to be? 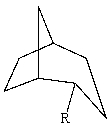
A)axial
B)equatorial
C)endo
D)exo
E)trans

A)axial
B)equatorial
C)endo
D)exo
E)trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
45
Which is not a proper resonance structure for 1,3-butadiene?
A)CH2=CH-CH=CH2
B)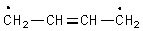
C)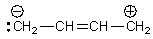
D)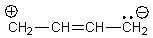
E)All of these answer choices are correct.
A)CH2=CH-CH=CH2
B)
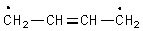
C)
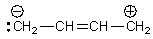
D)
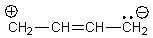
E)All of these answer choices are correct.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
46
Select the structure of the conjugated diene. 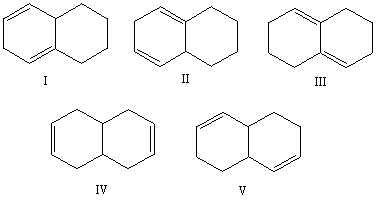
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following dienes is a cumulated diene?
A)CH2=CHCH2CH2CH=CH2
B)CH2=CHCH=CHCH2CH3
C)CH3CH=C=CHCH2CH3
D)CH3CH=CHCH=CHCH3
E)CH3CH=CHCH2CH=CH2
A)CH2=CHCH2CH2CH=CH2
B)CH2=CHCH=CHCH2CH3
C)CH3CH=C=CHCH2CH3
D)CH3CH=CHCH=CHCH3
E)CH3CH=CHCH2CH=CH2

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
48
Which pair does not represent a pair of resonance structures? 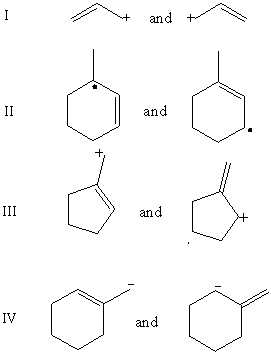
A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
49
Select the structure of the conjugated diene. 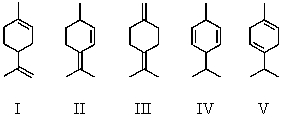
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
50
The substituent R on the bicyclic compound shown is considered to be? 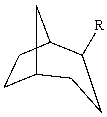
A)axial
B)equatorial
C)endo
D)exo
E)trans

A)axial
B)equatorial
C)endo
D)exo
E)trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
51
Which is not an example of resonance? 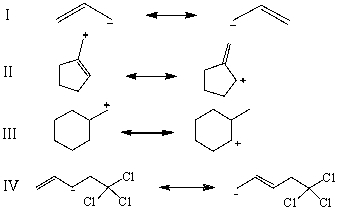
A)I
B)II
C)III
D)IV
E)None of these choices are examples of resonance.

A)I
B)II
C)III
D)IV
E)None of these choices are examples of resonance.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
52
Which pair does not represent a pair of resonance structures? 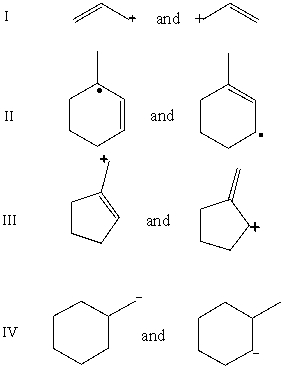
A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
53
A correct IUPAC name of the compound below is: 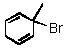
A)1-Bromo-1-methyl-2,5-cyclohexadiene
B)3-Bromo-3-methyl-1,4-cyclohexadiene
C)6-Bromo-6-methyl-1,4-cyclohexadiene
D)2-Bromo-2-methyl-1,3-cyclohexadiene
E)None of these choices.

A)1-Bromo-1-methyl-2,5-cyclohexadiene
B)3-Bromo-3-methyl-1,4-cyclohexadiene
C)6-Bromo-6-methyl-1,4-cyclohexadiene
D)2-Bromo-2-methyl-1,3-cyclohexadiene
E)None of these choices.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
54
Which pair does not represent a pair of resonance structures? 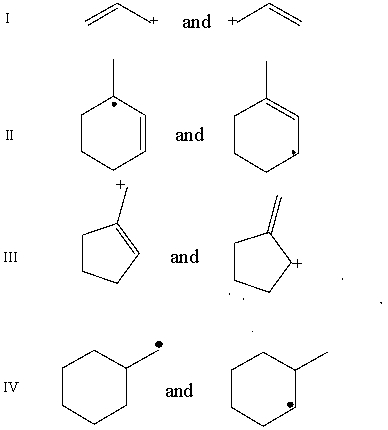
A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
55
Select the structure(s)in which the multiple bonds are conjugated. 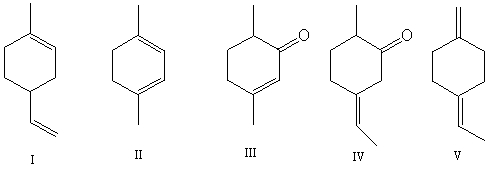
A)I and II
B)II and III
C)III and IV
D)I,II and V
E)V

A)I and II
B)II and III
C)III and IV
D)I,II and V
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
56
Select the structure(s)in which the multiple bonds are conjugated. 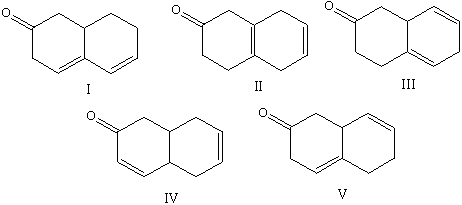
A)I and IV
B)II and III
C)III and V
D)I,II and III
E)V

A)I and IV
B)II and III
C)III and V
D)I,II and III
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
57
Which pair does not represent a pair of resonance structures? 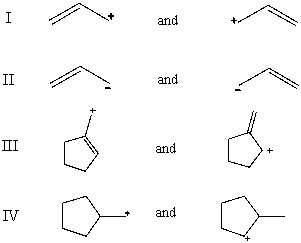
A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

A)I
B)II
C)III
D)IV
E)All of these choices represent pairs of resonance structures.

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
58
Select the structure(s)of the conjugated diene(s). 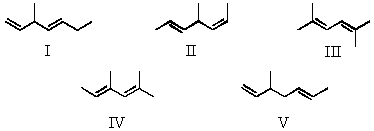
A)I and II
B)II and III
C)III and IV
D)I,II and V
E)V

A)I and II
B)II and III
C)III and IV
D)I,II and V
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
59
Select the structure of the isolated diene. 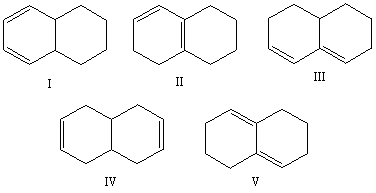
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
60
What is an IUPAC name for 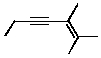
A)2,3-methylhept-2-en-4-yne
B)1,1,3-trimethylhex-3-yn-1-ene
C)1,1,3-trimethyl-3-hexyn-1-ene
D)2,3-dimethyl-2-hepten-4-yne
E)5,6-dimethyl-5-hepten-3-yne
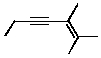
A)2,3-methylhept-2-en-4-yne
B)1,1,3-trimethylhex-3-yn-1-ene
C)1,1,3-trimethyl-3-hexyn-1-ene
D)2,3-dimethyl-2-hepten-4-yne
E)5,6-dimethyl-5-hepten-3-yne

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
61
The substituent R on the bicyclic compound shown is considered to be? 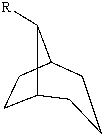
A)axial
B)equatorial
C)endo
D)exo
E)trans

A)axial
B)equatorial
C)endo
D)exo
E)trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
62
Which compound would have the shortest carbon-carbon single bond?
A)CH C-CH=CH-CH2-CH3
B)CH2=CH-CH=CH-CH3
C)HC C-CH2-C C-CH3
D)CH2=CH-C C-CH2-CH3
E)CH3-C C-C C-CH3
A)CH C-CH=CH-CH2-CH3
B)CH2=CH-CH=CH-CH3
C)HC C-CH2-C C-CH3
D)CH2=CH-C C-CH2-CH3
E)CH3-C C-C C-CH3

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
63
Which carbon-carbon bond in the following compound would you expect to be shortest? 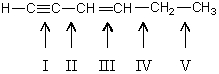
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
64
Which alkene would you expect to have the highest heat of hydrogenation? 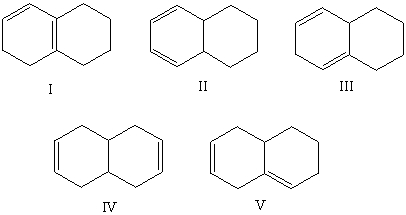
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
65
The substituent R on the bicyclic compound shown is considered to be? 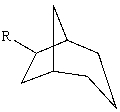
A)axial
B)equatorial
C)endo
D)exo
E)trans

A)axial
B)equatorial
C)endo
D)exo
E)trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
66
Which alkene would you expect to have the smallest heat of hydrogenation?
A)
B)
C)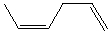
D)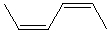
E)
A)

B)

C)
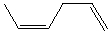
D)
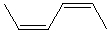
E)


Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
67
Which alkene would you expect to have the lowest heat of hydrogenation? 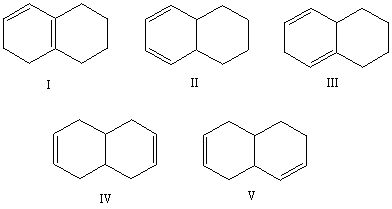
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
68
The substituent R on the bicyclic compound shown is considered to be? 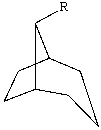
A)axial
B)equatorial
C)endo
D)exo
E)trans

A)axial
B)equatorial
C)endo
D)exo
E)trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
69
Which carbon-carbon bond in the following compound would you expect to be longest? 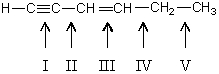
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
70
The substituent R on the bicyclic compound shown is considered to be? 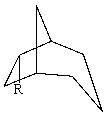
A)axial
B)equatorial
C)endo
D)exo
E)Trans

A)axial
B)equatorial
C)endo
D)exo
E)Trans

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
71
Which compound would have a UV absorption band at longest wavelength? 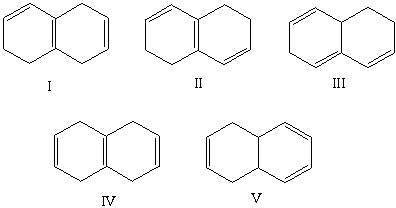
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
72
Which compound would have the shortest carbon-carbon single bond?
A)CH3-CH3
B)CH2=CH-CH3
C)HC C-C CH
D)CH2=CH-C CH
E)CH2=CH-CH=CH2
A)CH3-CH3
B)CH2=CH-CH3
C)HC C-C CH
D)CH2=CH-C CH
E)CH2=CH-CH=CH2

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
73
What is an IUPAC name for this triene? 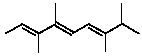
A)(2E,4Z,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene
B)(2Z,4E,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene
C)(2E,4Z,6E)-2,3,6,7-tetramethyl-3,5,7-Nonatriene
D)(2E,4Z,6E)-2,3,6,7-tetramethyl-3,5,7-nonatriene
E)(2E,4E,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene

A)(2E,4Z,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene
B)(2Z,4E,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene
C)(2E,4Z,6E)-2,3,6,7-tetramethyl-3,5,7-Nonatriene
D)(2E,4Z,6E)-2,3,6,7-tetramethyl-3,5,7-nonatriene
E)(2E,4E,6E)-3,4,7,8-tetramethyl-2,4,6-Nonatriene

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
74
Which alkene would you expect to have the lowest heat of hydrogenation? 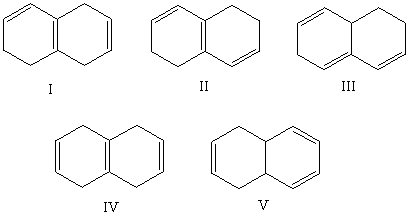
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
75
Which alkene would you expect to have the lowest heat of hydrogenation? 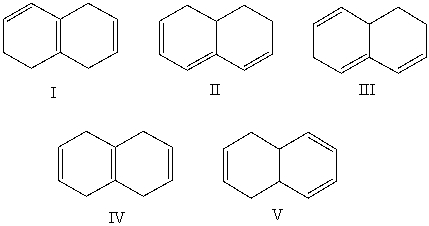
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
76
Which compound would have a UV absorption band at longest wavelength? 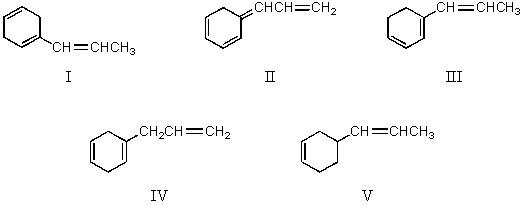
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
77
Which compound would have a UV absorption band at longest wavelength? 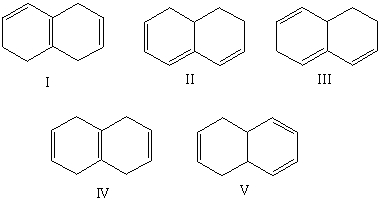
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
78
Which alkene would you expect to have the smallest heat of hydrogenation?
A)CH2=CHCH2CH2CH=CH2
B)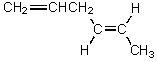
C)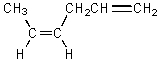
D)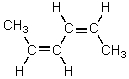
E)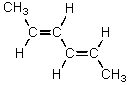
A)CH2=CHCH2CH2CH=CH2
B)

C)

D)

E)


Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
79
What is an IUPAC name for this triene? 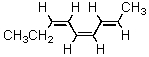
A)(2E,4Z,6E)-2,4,6-Nonatriene
B)(2Z,4E,6Z)-2,4,6-Nonatriene
C)(2E,4Z,6Z)-2,4,6-Nonatriene
D)(3Z,5Z,7E)-3,5,7-Nonatriene
E)(3Z,5E,7E)-3,5,7-Nonatriene

A)(2E,4Z,6E)-2,4,6-Nonatriene
B)(2Z,4E,6Z)-2,4,6-Nonatriene
C)(2E,4Z,6Z)-2,4,6-Nonatriene
D)(3Z,5Z,7E)-3,5,7-Nonatriene
E)(3Z,5E,7E)-3,5,7-Nonatriene

Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck
80
Which alkene would you expect to have the highest heat of hydrogenation?
A)
B)
C)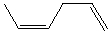
D)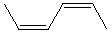
E)
A)

B)

C)
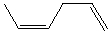
D)
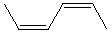
E)


Unlock Deck
Unlock for access to all 201 flashcards in this deck.
Unlock Deck
k this deck


