Deck 17: Carboxylic Acids and Their Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/213
Play
Full screen (f)
Deck 17: Carboxylic Acids and Their Derivatives
1
A correct name for 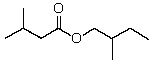 is:
is:
A)2-Methylbutyl 2-methylbutanoate
B)2-Methylbutyl 3-methylbutanoate
C)3-Methylbutyl isovalerate
D)Isopentyl isovalerate
E)Isopentyl isobutyrate
 is:
is:A)2-Methylbutyl 2-methylbutanoate
B)2-Methylbutyl 3-methylbutanoate
C)3-Methylbutyl isovalerate
D)Isopentyl isovalerate
E)Isopentyl isobutyrate
2-Methylbutyl 3-methylbutanoate
2
The correct structure for 2-methylbutyl methanoate is: 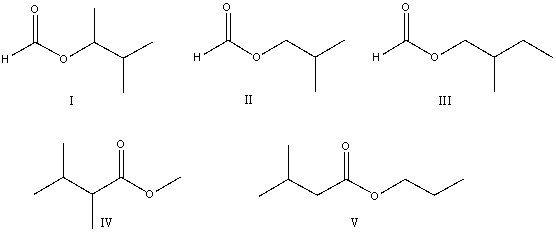
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
III
3
The correct structure for methyl bicyclo[2.2.2]octane-1-carboxylate is: ![<strong>The correct structure for methyl bicyclo[2.2.2]octane-1-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_f399_9180_970629f1b5ed_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for methyl bicyclo[2.2.2]octane-1-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_f399_9180_970629f1b5ed_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
IV
4
What is the IUPAC name for 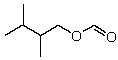
A)"2,3-Dimethylbutyl acetate-"
B)"2,3-Dimethyl-4-oxoethanal"
C)"2,3-Dimethylbutyl methanoate"
D)"2,3-Dimethylbutyl methylate"
E)"2,3-Dimethylbutyl formylate"

A)"2,3-Dimethylbutyl acetate-"
B)"2,3-Dimethyl-4-oxoethanal"
C)"2,3-Dimethylbutyl methanoate"
D)"2,3-Dimethylbutyl methylate"
E)"2,3-Dimethylbutyl formylate"

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
5
The correct structure for methyl bicyclo[2.2.2]octane-2-carboxylate is: ![<strong>The correct structure for methyl bicyclo[2.2.2]octane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc87_9180_cf0ca8eec15d_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for methyl bicyclo[2.2.2]octane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc87_9180_cf0ca8eec15d_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
6
Which compound would be the strongest acid?
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the best name for the following compound? 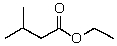
A)Isobutyl ethanoate
B)Ethyl isopropanoate
C)3-methylbutyl ethanoate
D)Ethoxy isobutyl ketone
E)Ethyl 3-methylbutanoate

A)Isobutyl ethanoate
B)Ethyl isopropanoate
C)3-methylbutyl ethanoate
D)Ethoxy isobutyl ketone
E)Ethyl 3-methylbutanoate

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
8
The correct structure for bicyclo[1.1.1]pentane-2-carboxylic acid is: ![<strong>The correct structure for bicyclo[1.1.1]pentane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a573_9180_9be89139f28a_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for bicyclo[1.1.1]pentane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a573_9180_9be89139f28a_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name for 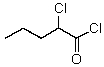
A)" -Chlorovaleryl chloride"
B)"2-Chloropentanoyl chloride"
C)"1-Chloropentanoyl chloride"
D)"1,2-Dichloropentanal"
E)"1-Chloro-1-butanecarbonyl chloride"

A)" -Chlorovaleryl chloride"
B)"2-Chloropentanoyl chloride"
C)"1-Chloropentanoyl chloride"
D)"1,2-Dichloropentanal"
E)"1-Chloro-1-butanecarbonyl chloride"

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
10
The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is: ![<strong>The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a574_9180_17757316f816_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a574_9180_17757316f816_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
11
The correct structure for ethyl 3-methylbutanoate is: 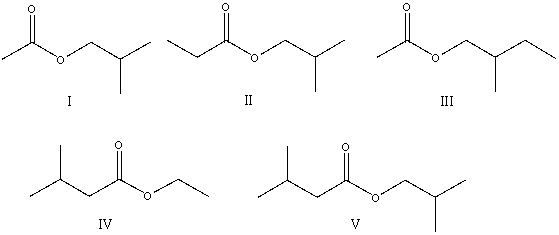
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following structures is 3,4-dimethylpentyl chloroformate? 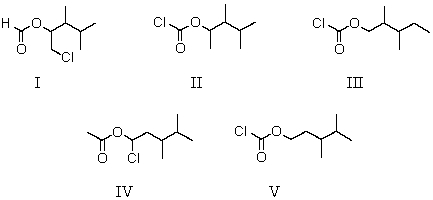
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
13
The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is: ![<strong>The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a575_9180_2bb0d7d8d503_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_a575_9180_2bb0d7d8d503_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
14
The correct structure for ethyl 3-methylbutanoate is: 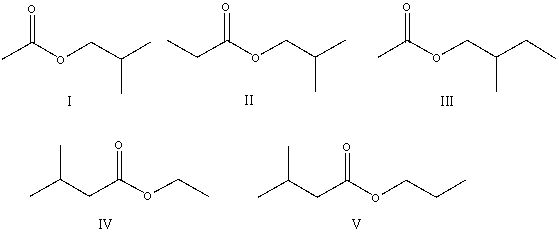
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
15
The correct structure for ethyl 2-chloropentanoyl chloride is: 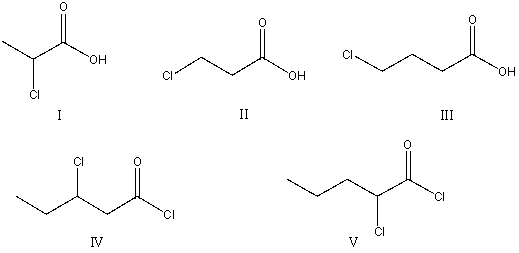
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
16
Which compound would be the weakest acid?
A)CHCl2CH2CH2CO2H
B)ClCH2CHClCH2CO2H
C)CH3CCl2CH2CO2H
D)CH3CHClCHClCO2H
E)CH3CH2CCl2CO2H
A)CHCl2CH2CH2CO2H
B)ClCH2CHClCH2CO2H
C)CH3CCl2CH2CO2H
D)CH3CHClCHClCO2H
E)CH3CH2CCl2CO2H

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following structures is N-benzyl-N-propyl-2,3-dimethylbutanamide? 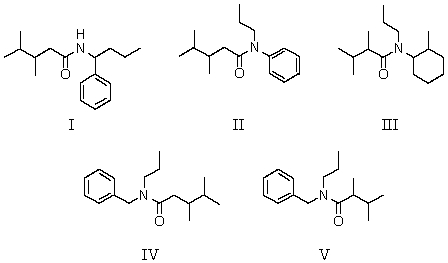
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
18
Which compound would be the strongest acid?
A)CHCl2CH2CH2CO2H
B)ClCH2CHClCH2CO2H
C)CH3CCl2CH2CO2H
D)CH3CHClCHClCO2H
E)CH3CH2CCl2CO2H
A)CHCl2CH2CH2CO2H
B)ClCH2CHClCH2CO2H
C)CH3CCl2CH2CO2H
D)CH3CHClCHClCO2H
E)CH3CH2CCl2CO2H

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
19
The correct structure for methyl bicyclo[1.1.0]butane-2-carboxylate is: ![<strong>The correct structure for methyl bicyclo[1.1.0]butane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc88_9180_a3aec7fda696_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for methyl bicyclo[1.1.0]butane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc88_9180_a3aec7fda696_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
20
The correct structure for methyl bicyclo[1.1.1]pentane-2-carboxylate is: ![<strong>The correct structure for methyl bicyclo[1.1.1]pentane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc86_9180_d553e2af500b_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V
![<strong>The correct structure for methyl bicyclo[1.1.1]pentane-2-carboxylate is: </strong> A)I B)II C)III D)IV E)V](https://storage.examlex.com/TB5902/11eaa4bc_3e0d_cc86_9180_d553e2af500b_TB5902_00.jpg)
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following would be the strongest acid? 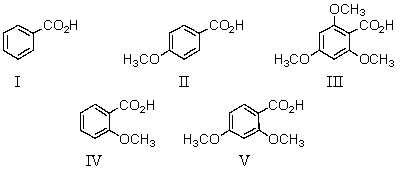
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following sequences are the compounds listed in order of decreasing acidity?
A)CH3COOH > H2O > CH3CH2OH > HC CH > NH3
B)CH3CH2OH > CH3COOH > H2O > HC CH > NH3
C)CH3COOH > CH3CH2OH > H2O > NH3 > HC CH
D)H2O > CH3COOH > CH3CH2OH > HC CH > NH3
E)CH3CH2OH > H2O > CH3COOH > HC CH > NH3
A)CH3COOH > H2O > CH3CH2OH > HC CH > NH3
B)CH3CH2OH > CH3COOH > H2O > HC CH > NH3
C)CH3COOH > CH3CH2OH > H2O > NH3 > HC CH
D)H2O > CH3COOH > CH3CH2OH > HC CH > NH3
E)CH3CH2OH > H2O > CH3COOH > HC CH > NH3

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following would be the weakest acid? 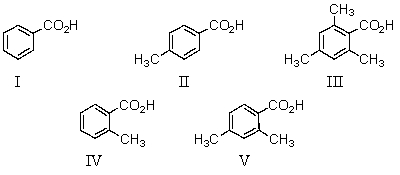
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound would be expected to have the highest pKa?
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
25
Which compound would be the weakest acid?
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound would be the strongest acid?
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
27
Which compound would be expected to have the lowest pKa?
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following would be the strongest acid? 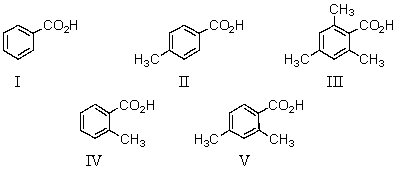
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following would be the weakest acid? 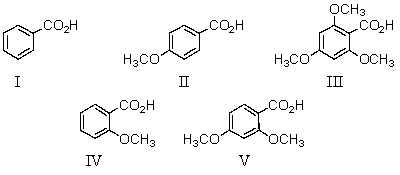
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
30
In which of the following sequences are the compounds listed in order of decreasing acidity?
A)PhCOOH > H2O > PhOH > PhCH2OH > PhH
B)PhCOOH > PhOH > H2O > PhCH2OH > PhH
C)PhH > H2O > PhOH > PhCH2OH > PhCOOH
D)PhOH > H2O > PhCOOH > PhCH2OH > PhH
E)PhCOOH > H2O > PhOH > PhH > PhCH2OH
A)PhCOOH > H2O > PhOH > PhCH2OH > PhH
B)PhCOOH > PhOH > H2O > PhCH2OH > PhH
C)PhH > H2O > PhOH > PhCH2OH > PhCOOH
D)PhOH > H2O > PhCOOH > PhCH2OH > PhH
E)PhCOOH > H2O > PhOH > PhH > PhCH2OH

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound would be the weakest acid?
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound would be expected to have the lowest pKa?
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid
A)4,4-dichlorobutanoic acid
B)3,4-dichlorobutanoic acid
C)3,3-dichlorobutanoic acid
D)2,3-dichlorobutanoic acid
E)2,2-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following would be the strongest acid? 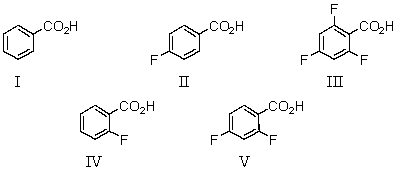
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would be the strongest acid? 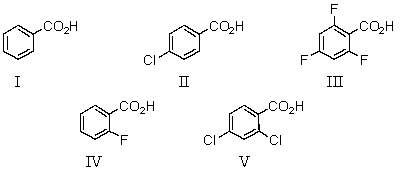
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following would be the weakest acid? 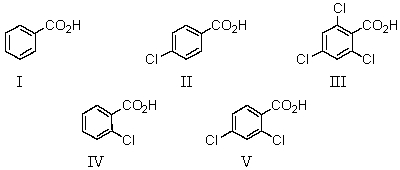
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following would be the strongest acid? 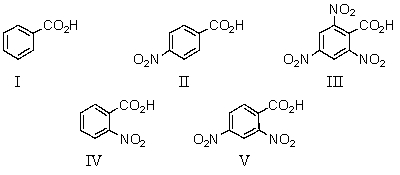
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
37
Which compound would be expected to have the highest pKa?
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol
A)water
B)acetic acid
C)ethane
D)acetylene
E)ethanol

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following would be the strongest acid? 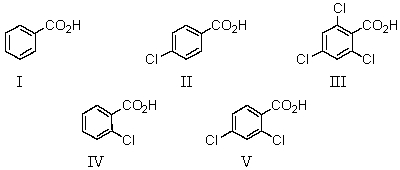
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
39
In which of the following sequences are the compounds listed in order of decreasing acidity?
A)CH3COOH > H2O > PhOH > HC CH > NH3
B)PhOH > CH3COOH > H2O > HC CH > NH3
C)CH3COOH > PhOH > H2O > HC CH > NH3
D)H2O > CH3COOH > PhOH > HC CH > NH3
E)PhOH > H2O > CH3COOH > HC CH > NH3
A)CH3COOH > H2O > PhOH > HC CH > NH3
B)PhOH > CH3COOH > H2O > HC CH > NH3
C)CH3COOH > PhOH > H2O > HC CH > NH3
D)H2O > CH3COOH > PhOH > HC CH > NH3
E)PhOH > H2O > CH3COOH > HC CH > NH3

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
40
In which of the following sequences are the compounds listed in order of increasing acidity?
A)NH3 < HC CH < CH3CH2OH < H2O < CH3COOH
B)CH3CH2OH < NH3 < H2O < HC CH < CH3COOH
C)CH3COOH < CH3CH2OH < H2O < NH3 < HC CH
D)H2O < CH3COOH < CH3CH2OH < HC CH < NH3
E)NH3 < H2O < CH3COOH < HC CH < CH3CH2OH
A)NH3 < HC CH < CH3CH2OH < H2O < CH3COOH
B)CH3CH2OH < NH3 < H2O < HC CH < CH3COOH
C)CH3COOH < CH3CH2OH < H2O < NH3 < HC CH
D)H2O < CH3COOH < CH3CH2OH < HC CH < NH3
E)NH3 < H2O < CH3COOH < HC CH < CH3CH2OH

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following acids would be expected to have the largest value for pKa?
A)4-bromobutanoic acid
B)4-chlorobutanoic acid
C)4,4-dichlorobutanoic acid
D)4-bromo-4-iodobutanoic acid
E)4-bromo-4,4-dichlorobutanoic acid
A)4-bromobutanoic acid
B)4-chlorobutanoic acid
C)4,4-dichlorobutanoic acid
D)4-bromo-4-iodobutanoic acid
E)4-bromo-4,4-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following acids would be expected to have the smallest value for pKa?
A)Acetic acid
B)Ethanol
C)Phenol
D)Acetone
E)Water
A)Acetic acid
B)Ethanol
C)Phenol
D)Acetone
E)Water

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
43
In which of the following are the compounds listed in order of decreasing acidity?
A)CH3CO2H > CH3CH2OH > C6H5OH > H2O
B)C6H5OH > CH3CO2H > H2O > CH3CH2OH
C)CH3CO2H > H2O > C6H5OH > CH3CH2OH
D)H2O > CH3CO2H > C6H5OH > CH3CH2OH
E)None of these choices.
A)CH3CO2H > CH3CH2OH > C6H5OH > H2O
B)C6H5OH > CH3CO2H > H2O > CH3CH2OH
C)CH3CO2H > H2O > C6H5OH > CH3CH2OH
D)H2O > CH3CO2H > C6H5OH > CH3CH2OH
E)None of these choices.

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following acids would have the smallest value for pKa1?
A)HOOCCH2CH2CH2CH2CH2COOH
B)HOOCCH2CH2CH2CH2COOH
C)HOOCCH2CH2CH2COOH
D)HOOCCH2CH2COOH
E)HOOCCH2COOH
A)HOOCCH2CH2CH2CH2CH2COOH
B)HOOCCH2CH2CH2CH2COOH
C)HOOCCH2CH2CH2COOH
D)HOOCCH2CH2COOH
E)HOOCCH2COOH

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
45
A compound has the molecular formula,C6H12O2.Its IR spectrum shows a strong absorption band near 1740 cm-1; its 1H NMR spectrum consists of two singlets,at 1.4 and 2.0.The most likely structure for this compound is: 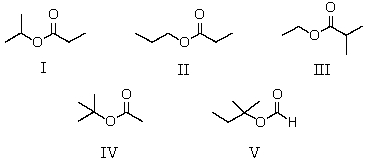
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
46
Which compound would be most acidic?
A)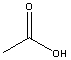
B)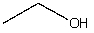
C)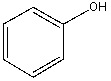
D)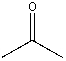
E)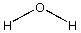
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following would be the strongest acid?
A)2,3-Dimethylhexanoic acid
B)3,3-Diiodopentanoic acid
C)3-Iodo-4-bromopentanoic acid
D)3-Chloro-4-bromohexanoic acid
E)2-Fluoro-4-bromopentanoic acid
A)2,3-Dimethylhexanoic acid
B)3,3-Diiodopentanoic acid
C)3-Iodo-4-bromopentanoic acid
D)3-Chloro-4-bromohexanoic acid
E)2-Fluoro-4-bromopentanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
48
A compound has the molecular formula C8H14O4.Its IR spectrum shows a strong absorption band near 1740 cm-1.Its 1H NMR spectrum consists of: triplet, 1.3
Singlet, 2.6
Quartet, 4.2
The most likely structure for the compound is: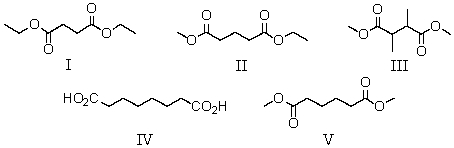
A)I
B)II
C)III
D)IV
E)V
Singlet, 2.6
Quartet, 4.2
The most likely structure for the compound is:

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following would be the weakest acid?
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following acids would have the smallest value for pKa?
A)BrCH2CH2CH2COOH
B)ClCH2CH2CH2COOH
C)Cl2CHCH2CH2COOH
D)ICHBrCH2CH2COOH
E)BrCCl2CH2CH2COOH
A)BrCH2CH2CH2COOH
B)ClCH2CH2CH2COOH
C)Cl2CHCH2CH2COOH
D)ICHBrCH2CH2COOH
E)BrCCl2CH2CH2COOH

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
51
A compound has the molecular formula,C6H12O2.Its IR spectrum shows a strong absorption band near 1740 cm-1; its 1H NMR spectrum consists of two singlets,at 1.2 and 3.6.The most likely structure for this compound is: 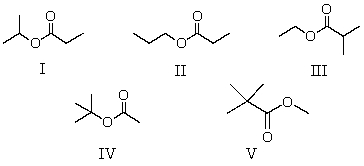
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following acids would be expected to have the smallest value for pKa?
A)2,3-Dimethylhexanoic acid
B)3,3-Diiodopentanoic acid
C)3-Iodo-4-bromopentanoic acid
D)3-Chloro-4-bromohexanoic acid
E)2-Fluoro-4-bromopentanoic acid
A)2,3-Dimethylhexanoic acid
B)3,3-Diiodopentanoic acid
C)3-Iodo-4-bromopentanoic acid
D)3-Chloro-4-bromohexanoic acid
E)2-Fluoro-4-bromopentanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
53
The IR spectrum of a compound exhibits a broad absorption band at 2500-3000 cm-1 and a sharp band at 1710 cm-1.Which of these compounds could it be?
A)1-Butanol
B)Propyl acetate
C)Butanoic acid
D)Acetyl chloride
E)Acetic anhydride
A)1-Butanol
B)Propyl acetate
C)Butanoic acid
D)Acetyl chloride
E)Acetic anhydride

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
54
Which compound would be most acidic?
A)Acetic acid
B)Ethanol
C)Phenol
D)Acetone
E)Water
A)Acetic acid
B)Ethanol
C)Phenol
D)Acetone
E)Water

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following acids would have the largest value for pKa?
A)BrCH2CH2CH2COOH
B)ClCH2CH2CH2COOH
C)Cl2CHCH2CH2COOH
D)ICHBrCH2CH2COOH
E)BrCCl2CH2CH2COOH
A)BrCH2CH2CH2COOH
B)ClCH2CH2CH2COOH
C)Cl2CHCH2CH2COOH
D)ICHBrCH2CH2COOH
E)BrCCl2CH2CH2COOH

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following acids would be expected to have the smallest value for pKa?
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following acids would be expected to have the largest value for pKa?
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following acids would be expected to have the smallest value for pKa?
A)4-bromobutanoic acid
B)4-chlorobutanoic acid
C)4,4-dichlorobutanoic acid
D)4-bromo-4-iodobutanoic acid
E)4-bromo-4,4-dichlorobutanoic acid
A)4-bromobutanoic acid
B)4-chlorobutanoic acid
C)4,4-dichlorobutanoic acid
D)4-bromo-4-iodobutanoic acid
E)4-bromo-4,4-dichlorobutanoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following would be the strongest acid?
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid
A)Benzoic acid
B)4-Nitrobenzoic acid
C)4-Methylbenzoic acid
D)4-Methoxybenzoic acid
E)4-Iodobenzoic acid

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
60
In which of the following are the compounds listed in order of decreasing acidity?
A)CH3CO2H > CH3CH2OH > C6H5OH > H2O
B)C6H5OH > CH3CO2H > H2O > CH3CH2OH
C)CH3CO2H > H2O > C6H5OH > CH3CH2OH
D)H2O > CH3CO2H > C6H5OH > CH3CH2OH
E)CH3CO2H > C6H5OH > CH3CH2OH > H2O
A)CH3CO2H > CH3CH2OH > C6H5OH > H2O
B)C6H5OH > CH3CO2H > H2O > CH3CH2OH
C)CH3CO2H > H2O > C6H5OH > CH3CH2OH
D)H2O > CH3CO2H > C6H5OH > CH3CH2OH
E)CH3CO2H > C6H5OH > CH3CH2OH > H2O

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
61
What would be the product of the following reaction? 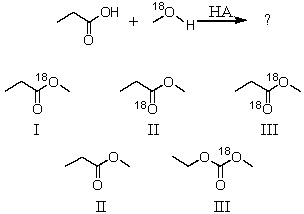 \
\
A)I
B)II
C)III
D)IV
E)V
 \
\A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
62
What is the expected product,A,of the following reaction sequence? 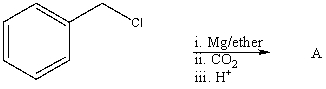
A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2OSO3H
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2

A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2OSO3H
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
63
What would be the final organic product of the following reaction? 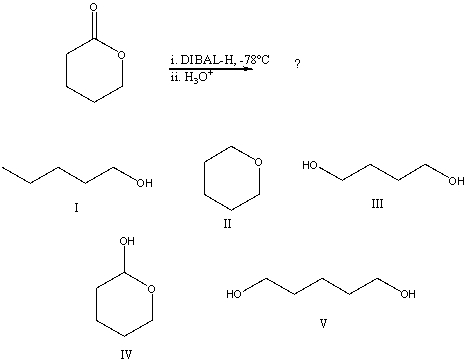
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
64
What would be the final organic product of the following reaction? 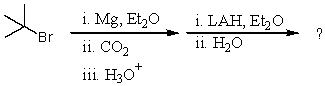
A)(CH3)3CCO2H
B)(CH3)3CCOCH3
C)(CH3)3CCH2OH
D)(CH3)3COCH3
E)(CH3)3CCO2CH3
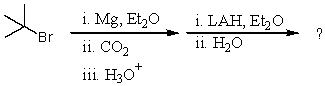
A)(CH3)3CCO2H
B)(CH3)3CCOCH3
C)(CH3)3CCH2OH
D)(CH3)3COCH3
E)(CH3)3CCO2CH3

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
65
What would be the final organic product of the following reaction? 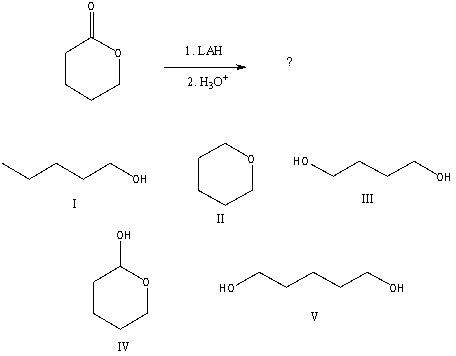
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
66
What is the expected product,A,of the following reaction sequence? 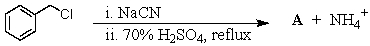
A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2OSO3H
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2
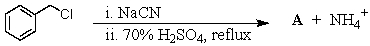
A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2OSO3H
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
67
Predict the major organic product of the reaction sequence, 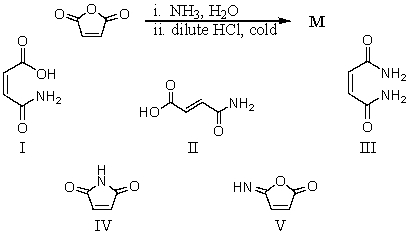
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
68
Predict the major organic product of the reaction sequence, 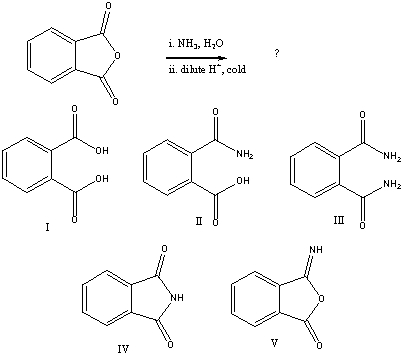
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
69
Predict the major organic product of the reaction sequence, 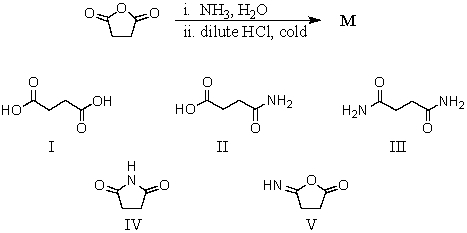
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
70
A compound with the molecular formula C18H18O4 has a 1H NMR spectrum that consists of: singlet, 2.7
Singlet, 3.1
Multiplet, 7.3
The IR spectrum shows a strong absorption band near 1750 cm-1.The most likely structure for the compound is:
A)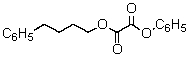
B)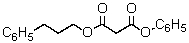
C)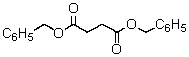
D)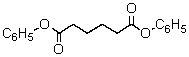
E)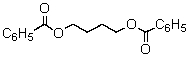
Singlet, 3.1
Multiplet, 7.3
The IR spectrum shows a strong absorption band near 1750 cm-1.The most likely structure for the compound is:
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
71
What would be the final organic product of the following reaction? 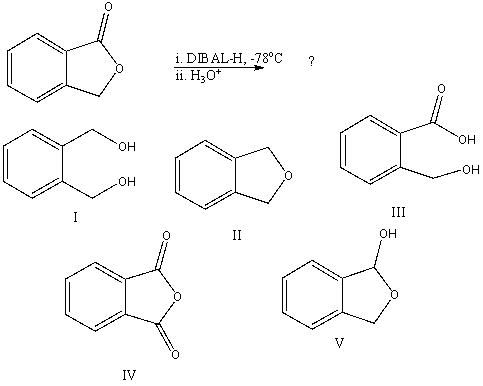
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
72
What is the product of this reaction? 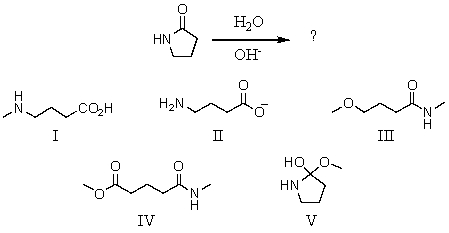
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
73
What is the reactant of the following reaction sequence? 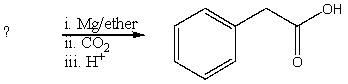
A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2Cl
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2

A)HCO2CH2C6H5
B)C6H5CH2COOH
C)C6H5CH2Cl
D)C6H5CHClCOOH
E)O=C(CH2C6H5)2

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
74
The product,Z,of the following sequence of reactions is which compound? 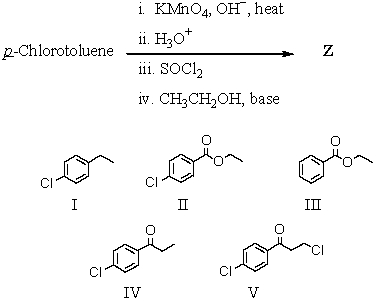
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the major organic product of the reaction sequence below: 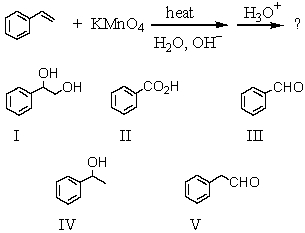
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
76
What would be the final organic product of the following reaction? 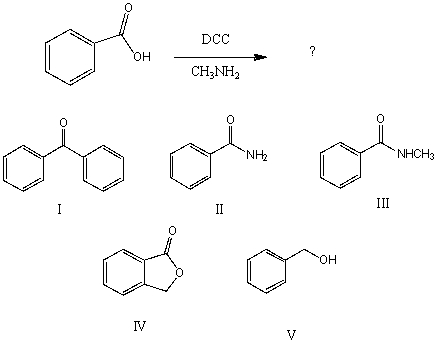
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
77
The product of the following reaction is: 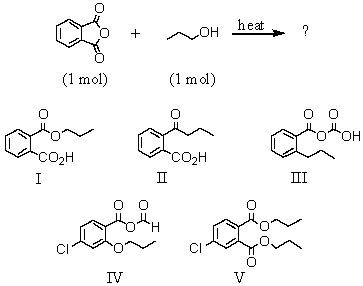
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
78
A compound with the molecular formula C5H10O2 gave the following 1H NMR spectrum: triplet, 0.90
Multiplet, 1.60
Singlet, 1.95
Triplet, 3.95
The IR spectrum showed a strong absorption band near 1740 cm-1.The most likely structure for the compound is:
A)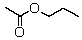
B)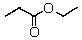
C)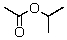
D)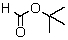
E)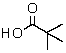
Multiplet, 1.60
Singlet, 1.95
Triplet, 3.95
The IR spectrum showed a strong absorption band near 1740 cm-1.The most likely structure for the compound is:
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
79
What is the product of this reaction? 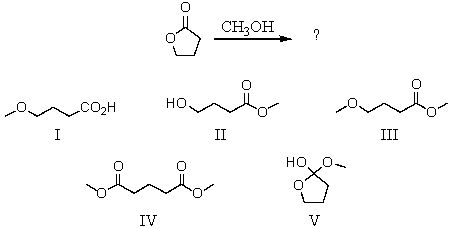
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck
80
What would be the final organic product of the following reaction? 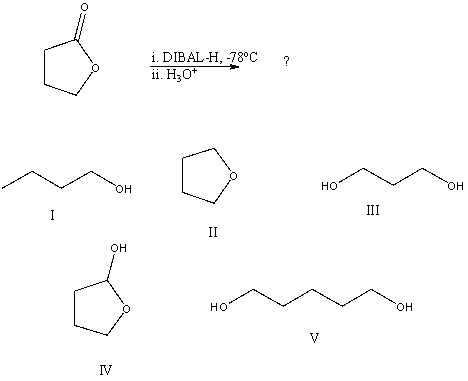
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 213 flashcards in this deck.
Unlock Deck
k this deck


