Deck 23: Lipids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 23: Lipids
1
What of the following solvents would dissolve triacylglycerols?
A)Water and chloroform
B)Acetic acid and water
C)Chloroform and diethyl ether
D)Aqueous NaOH and acetic acid
E)They are soluble in water only
A)Water and chloroform
B)Acetic acid and water
C)Chloroform and diethyl ether
D)Aqueous NaOH and acetic acid
E)They are soluble in water only
Chloroform and diethyl ether
2
What of the following will fill properly the blanks in the sentence. Lipids are classified according to ___,and they include ___ structural types.
A)their structure; two
B)their melting temperature; fats and oils.
C)their melting point; triglycerides and phospholipids.
D)the physical operation done to isolate them; a variety of
E)their structure; only terpene and steroid.
A)their structure; two
B)their melting temperature; fats and oils.
C)their melting point; triglycerides and phospholipids.
D)the physical operation done to isolate them; a variety of
E)their structure; only terpene and steroid.
the physical operation done to isolate them; a variety of
3
Which of the following statements regarding triacylglycerols is not true?
A)Some undergo autoxidation with oxygen from air.
B)They are solid if they do not have alkene groups.
C)They form lipid bilayers.
D)They form micelles in water.
E)Two of these choices are false statements.
A)Some undergo autoxidation with oxygen from air.
B)They are solid if they do not have alkene groups.
C)They form lipid bilayers.
D)They form micelles in water.
E)Two of these choices are false statements.
Two of these choices are false statements.
4
Of the saturated fatty acids found in fats and oils,this one normally is the most abundant:
A)Capric acid
B)Lauric acid
C)Myristic acid
D)Palmitic acid
E)Stearic acid
A)Capric acid
B)Lauric acid
C)Myristic acid
D)Palmitic acid
E)Stearic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
Rank the following fatty acids according to increasing melting point. I: CH3(CH2)16CO2H
II: CH3(CH2)7CH=CH(CH2)7CO2H (cis)
III: CH3(CH2)7CH=CH(CH2)7CO2H (trans)
IV: HO2C(CH2)7CH=CHCH2CH=CH(CH2)7CH3 (cis,cis)
A)I < II < III < IV
B)IV < III < II < I
C)II < IV < III < I
D)IV < II < III < I
E)IV < I < III < II
II: CH3(CH2)7CH=CH(CH2)7CO2H (cis)
III: CH3(CH2)7CH=CH(CH2)7CO2H (trans)
IV: HO2C(CH2)7CH=CHCH2CH=CH(CH2)7CH3 (cis,cis)
A)I < II < III < IV
B)IV < III < II < I
C)II < IV < III < I
D)IV < II < III < I
E)IV < I < III < II

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
What is a mixed triacylglycerol?
A)It is a lipid where a terpene is esterifying glycerol
B)It is a triester of glycerol where the acyl groups are identical
C)It is a triester formed by glycerol and acetic acid
D)It is a triester of glycerol where the fatty acids are not all identical
E)It is an ester between glycerol and cholesterol
A)It is a lipid where a terpene is esterifying glycerol
B)It is a triester of glycerol where the acyl groups are identical
C)It is a triester formed by glycerol and acetic acid
D)It is a triester of glycerol where the fatty acids are not all identical
E)It is an ester between glycerol and cholesterol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements regarding triacylglycerols is true?
A)They form micelles when mixed with water.
B)They are liquid if they are unsaturated.
C)They are solid if they have alkyne bonds.
D)They can be used to wash dirty dishes
E)They are soluble in water.
A)They form micelles when mixed with water.
B)They are liquid if they are unsaturated.
C)They are solid if they have alkyne bonds.
D)They can be used to wash dirty dishes
E)They are soluble in water.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
What of the following salts are useful for washing fatty stains in hard water? 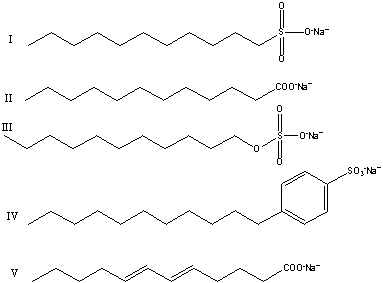
A)II and V
B)I,II and V
C)I,III and IV
D)I,II,III,and IV
E)I,II,III,IV and V

A)II and V
B)I,II and V
C)I,III and IV
D)I,II,III,and IV
E)I,II,III,IV and V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
What of the following structures is a biodegradable soap or detergent? 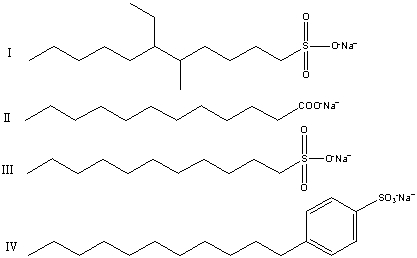
A)I,III and IV
B)Only I
C)II and III
D)III and IV
E)I and III

A)I,III and IV
B)Only I
C)II and III
D)III and IV
E)I and III

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements regarding triacylglycerols is not true?
A)They are synthesized in the body by adipocytes.
B)They have an odd number of carbon atoms.
C)They are solid if they do not have alkene groups.
D)Those which can be hydrogenated undergo a significant change in melting point upon hydrogenation.
E)They can be hydrolyzed to give glycerol and fatty acids.
A)They are synthesized in the body by adipocytes.
B)They have an odd number of carbon atoms.
C)They are solid if they do not have alkene groups.
D)Those which can be hydrogenated undergo a significant change in melting point upon hydrogenation.
E)They can be hydrolyzed to give glycerol and fatty acids.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements regarding triacylglycerols is not true?
A)They undergo alkaline hydrolysis to yield soaps.
B)They are liquid if they have alkene groups.
C)They are solid if they do not have one or more alkene groups.
D)They are soluble in water.
E)Some can be hydrogenated.
A)They undergo alkaline hydrolysis to yield soaps.
B)They are liquid if they have alkene groups.
C)They are solid if they do not have one or more alkene groups.
D)They are soluble in water.
E)Some can be hydrogenated.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
A simple triacylglycerol is one that:
A)Upon hydrolysis produces glycerol and three different fatty acids.
B)Upon hydrolysis produces glycerol and at least two different fatty acids.
C)Upon saponification gives glycerol and three fatty acid salts.
D)Upon saponification gives three esters.
E)Upon hydrolysis produces glycerol and three mol of a fatty acid.
A)Upon hydrolysis produces glycerol and three different fatty acids.
B)Upon hydrolysis produces glycerol and at least two different fatty acids.
C)Upon saponification gives glycerol and three fatty acid salts.
D)Upon saponification gives three esters.
E)Upon hydrolysis produces glycerol and three mol of a fatty acid.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Which is an untrue statement concerning the fatty acid moieties of naturally occurring triacylglycerols?
A)Generally,they possess an even number of carbon atoms.
B)Most have unbranched carbon chains.
C)The double bonds,when present,all are in the cis configuration.
D)Where two or three double bonds are present in the same fatty acid moiety,they comprise a conjugated system.
E)The fatty acid moieties in a particular triacylglycerol usually are different.
A)Generally,they possess an even number of carbon atoms.
B)Most have unbranched carbon chains.
C)The double bonds,when present,all are in the cis configuration.
D)Where two or three double bonds are present in the same fatty acid moiety,they comprise a conjugated system.
E)The fatty acid moieties in a particular triacylglycerol usually are different.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
What is an undesired effect of the partial hydrogenation of oils?
A)The oil gets very liquidly
B)The oil gets transformed into a brittle solid
C)The oil gets solid but the remaining double bonds are all in cis stereochemistry
D)Some double bonds are isomerized to trans stereochemistry
E)All the double bonds are hydrogenated and the fatty acids become fully saturated
A)The oil gets very liquidly
B)The oil gets transformed into a brittle solid
C)The oil gets solid but the remaining double bonds are all in cis stereochemistry
D)Some double bonds are isomerized to trans stereochemistry
E)All the double bonds are hydrogenated and the fatty acids become fully saturated

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
What of the following statements are true:
A)Fatty acids are linear and contain an even number of carbons.
B)Triacylglycerols contain ether functionalities.
C)Triacylglycerols are formed by long chain alcohols and glycerol.
D)Triacylglycerols containing unsaturated fatty acids have high melting temperature.
E)Two of these statements are true.
A)Fatty acids are linear and contain an even number of carbons.
B)Triacylglycerols contain ether functionalities.
C)Triacylglycerols are formed by long chain alcohols and glycerol.
D)Triacylglycerols containing unsaturated fatty acids have high melting temperature.
E)Two of these statements are true.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following would serve as the basis for a simple chemical test that would distinguish between stearic acid and oleic acid?
A)NaOH/H2O
B)NaHCO3/H2O
C)HCl/H2O
D)Ag(NH3)2+
E)Br2/CCl4
A)NaOH/H2O
B)NaHCO3/H2O
C)HCl/H2O
D)Ag(NH3)2+
E)Br2/CCl4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
Which is not a correct statement concerning naturally occurring triacylglycerols?
A)The greater the degree of unsaturation,the higher the melting point.
B)Saponification yields glycerol and a mixture of carboxylic acid salts.
C)Solid triacylglycerols are termed "fats."
D)Regardless of the exact nature of the R groups,such compounds are water-insoluble.
E)Such compounds frequently are called "triglycerides."
A)The greater the degree of unsaturation,the higher the melting point.
B)Saponification yields glycerol and a mixture of carboxylic acid salts.
C)Solid triacylglycerols are termed "fats."
D)Regardless of the exact nature of the R groups,such compounds are water-insoluble.
E)Such compounds frequently are called "triglycerides."

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
What is likely to be a natural fatty acid? 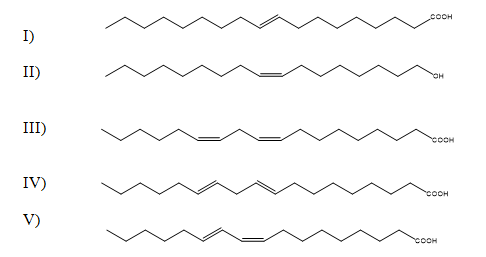
A)Compounds I and IV
B)Only compound III
C)Compounds II and III
D)Compounds IV and V
E)Only compound II

A)Compounds I and IV
B)Only compound III
C)Compounds II and III
D)Compounds IV and V
E)Only compound II

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
How could you convert an unsaturated fatty acid into a saturated fatty acid?
A)KMnO4,OH-,heat
B)OH-,H2O,heat; then H3O+
C)H2,Ni,pressure
D)H3O+,H2O,heat
E)O3; then Zn,HOAc
A)KMnO4,OH-,heat
B)OH-,H2O,heat; then H3O+
C)H2,Ni,pressure
D)H3O+,H2O,heat
E)O3; then Zn,HOAc

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these is a detergent?
A)CH3(CH2)16COO-Na+
B)[CH3(CH2)14COO-]2Ca2+
C)CH3(CH2)10CH2SO3-Na+
D)HOCH2CHOHCH2OH
E)CH3(CH2)14CH2SH
A)CH3(CH2)16COO-Na+
B)[CH3(CH2)14COO-]2Ca2+
C)CH3(CH2)10CH2SO3-Na+
D)HOCH2CHOHCH2OH
E)CH3(CH2)14CH2SH

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these reagents would not react with stearic acid?
A)H2,Ni
B)SOCl2
C)CH3MgI
D)NH3/H2O
E)LAH (lithium aluminum hydride)
A)H2,Ni
B)SOCl2
C)CH3MgI
D)NH3/H2O
E)LAH (lithium aluminum hydride)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would not be helpful in distinguishing between oleic acid,linoleic acid and linolenic acid?
A)Examine the stoichiometry of the reaction with Br2,CCl4
B)Examine the stoichiometry of complete catalytic hydrogenation.
C)Examine the products obtained after subjecting the sample to catalytic hydrogenation
D)Examine products obtained after subjecting the sample to: i)O3,CH2Cl2; ii)Zn,CH3CO2H
E)Examine the molecular weight of the acids.
A)Examine the stoichiometry of the reaction with Br2,CCl4
B)Examine the stoichiometry of complete catalytic hydrogenation.
C)Examine the products obtained after subjecting the sample to catalytic hydrogenation
D)Examine products obtained after subjecting the sample to: i)O3,CH2Cl2; ii)Zn,CH3CO2H
E)Examine the molecular weight of the acids.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
What would be the product of the following reaction sequence? 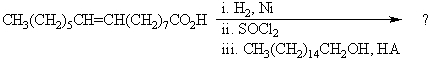
A)CH3(CH2)5CH=CH(CH2)14CH3
B)CH3(CH2)5COOCH2(CH2)14CH3 and CH3(CH2)5CH=CH(CH2)7COOCH2(CH2)14CH3
C)CH3(CH2)14COOCH2(CH2)14CH3
D)CH3(CH2)13CHClCOOCH2(CH2)14CH3
E)CH3(CH2)5CH=CH(CH2)7COOCH2(CH2)14CH3
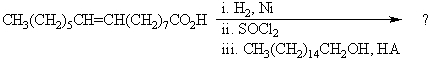
A)CH3(CH2)5CH=CH(CH2)14CH3
B)CH3(CH2)5COOCH2(CH2)14CH3 and CH3(CH2)5CH=CH(CH2)7COOCH2(CH2)14CH3
C)CH3(CH2)14COOCH2(CH2)14CH3
D)CH3(CH2)13CHClCOOCH2(CH2)14CH3
E)CH3(CH2)5CH=CH(CH2)7COOCH2(CH2)14CH3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
Which fatty acid is not likely to occur commonly in natural sources?
A)(Z)-11-Tetradecenoic acid
B)(Z)-9-Hexadecenoic acid
C)Hexadecanoic acid
D)(9Z,12Z)-9,12-octadecadienoic acid
E)(Z)-9-pentadecenoic acid
A)(Z)-11-Tetradecenoic acid
B)(Z)-9-Hexadecenoic acid
C)Hexadecanoic acid
D)(9Z,12Z)-9,12-octadecadienoic acid
E)(Z)-9-pentadecenoic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following could be used to prepare myristic acid,CH3(CH2)12COOH?
A)CH3(CH2)11CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)11CH2Br,Mg,(C2H5)2O; then CO2; then H3O+
C)CH3(CH2)13CHO,Ag2O,OH-; then H3O+
D)Two of these choices.
E)All of these choices.
A)CH3(CH2)11CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)11CH2Br,Mg,(C2H5)2O; then CO2; then H3O+
C)CH3(CH2)13CHO,Ag2O,OH-; then H3O+
D)Two of these choices.
E)All of these choices.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
The ozonolysis of a fatty acid produces these fragments: 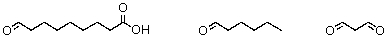 What is the identity of the fatty acid?
What is the identity of the fatty acid?
A)Stearic acid
B)Palmitoleic acid
C)Oleic acid
D)Linoleic acid
E)Linolenic acid
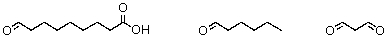 What is the identity of the fatty acid?
What is the identity of the fatty acid?A)Stearic acid
B)Palmitoleic acid
C)Oleic acid
D)Linoleic acid
E)Linolenic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
How could you synthesize stearolic acid,CH3(CH2)7C=C(CH2)7COOH from oleic acid,CH3(CH2)7CH=CH(CH2)7COOH?
A)Br2,CCl4; then 3 NaNH2,heat; then H3O+
B)Li,liq.NH3; then H3O+
C)H2,Pd
D)Peracid; then H3O+; then HA,H2O,heat
E)Excess HCl; then KOH,C2H5OH,heat
A)Br2,CCl4; then 3 NaNH2,heat; then H3O+
B)Li,liq.NH3; then H3O+
C)H2,Pd
D)Peracid; then H3O+; then HA,H2O,heat
E)Excess HCl; then KOH,C2H5OH,heat

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these reagents would not react with oleic acid?
A)H2,Ni
B)DIBAL-H (diisobutylaluminium hydride)
C)O3/CH2Cl2
D)NH3/H2O
E)LAH (lithium aluminium hydride)
A)H2,Ni
B)DIBAL-H (diisobutylaluminium hydride)
C)O3/CH2Cl2
D)NH3/H2O
E)LAH (lithium aluminium hydride)

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
Which fatty acid is likely to occur commonly in natural sources? 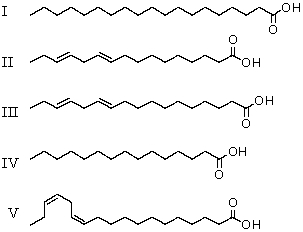
A)II
B)I and IV
C)II and III
D)II,III and IV
E)V

A)II
B)I and IV
C)II and III
D)II,III and IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following could be used to prepare stearic acid,CH3(CH2)16COOH?
A)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)15CH2Br,Mg,(C2H5)2O; then HCHO; then H3O+
C)CH3(CH2)16CH2Br,NaOH; then KMnO4,OH-,H2O; then H3O+
D)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat and CH3(CH2)15CH2Br,Mg,(C2H5)2O; then HCHO; then H3O+
E)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat and CH3(CH2)16CH2Br,NaOH; then KMnO4,OH-,H2O; then H3O+
A)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)15CH2Br,Mg,(C2H5)2O; then HCHO; then H3O+
C)CH3(CH2)16CH2Br,NaOH; then KMnO4,OH-,H2O; then H3O+
D)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat and CH3(CH2)15CH2Br,Mg,(C2H5)2O; then HCHO; then H3O+
E)CH3(CH2)15CH2Br,CN-,heat; then H3O+,heat and CH3(CH2)16CH2Br,NaOH; then KMnO4,OH-,H2O; then H3O+

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
Which fatty acid is not likely to occur commonly in natural sources?
A)CH3(CH2)12COOH
B)CH3(CH2)14COOH
C)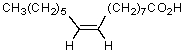
D)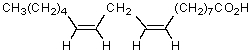
E)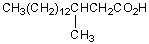
A)CH3(CH2)12COOH
B)CH3(CH2)14COOH
C)

D)

E)
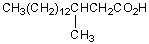

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
Which fatty acid is not likely to occur commonly in natural sources?
A)Tetradecanoic acid
B)Pentadecanoic acid
C)Hexadecanoic acid
D)(9Z,12Z)-octadeca-9,12-dienoic acid
E)(Z)-hexadeca-9-enoic acid
A)Tetradecanoic acid
B)Pentadecanoic acid
C)Hexadecanoic acid
D)(9Z,12Z)-octadeca-9,12-dienoic acid
E)(Z)-hexadeca-9-enoic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
How many isomers,including stereoisomers,exist for the triacylglycerol which,on saponification,gives glycerol,2 molar equivalents of palmitate and 1 molar equivalent of stearate?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
Which fatty acid is responsible for the putrid odor of rancid butter?
A)Valeric acid
B)Myristic acid
C)Stearic acid
D)Oleic acid
E)Butyric acid
A)Valeric acid
B)Myristic acid
C)Stearic acid
D)Oleic acid
E)Butyric acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
What would be the product,X,of the following reaction sequence? 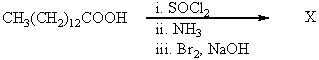
A)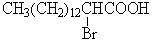
B)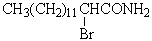
C)CH3(CH2)11CH2NH2
D)CH3(CH2)12CONH2
E)CH3(CH2)12COBr
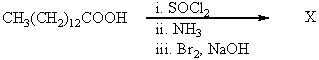
A)
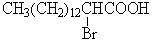
B)
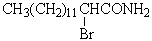
C)CH3(CH2)11CH2NH2
D)CH3(CH2)12CONH2
E)CH3(CH2)12COBr

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
The product of the following reaction sequence is nervonic acid.What is the structure of nervonic acid? 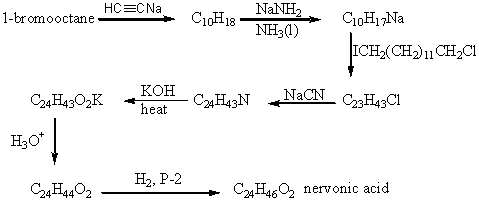
A)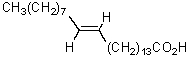
B)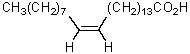
C)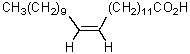
D)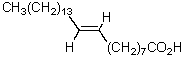
E)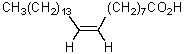

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following might be helpful in distinguishing between oleic acid and linoleic acid?
A)Examine the stoichiometry of the reaction with NaOH/H2O
B)Examine the stoichiometry of complete catalytic hydrogenation.
C)Examine products obtained after subjecting the sample to catalytic hydrogenation
D)Examine products obtained after subjecting the sample to: i)O3,CH2Cl2; ii)Zn,CH3CO2H
E)Two of these choices.
A)Examine the stoichiometry of the reaction with NaOH/H2O
B)Examine the stoichiometry of complete catalytic hydrogenation.
C)Examine products obtained after subjecting the sample to catalytic hydrogenation
D)Examine products obtained after subjecting the sample to: i)O3,CH2Cl2; ii)Zn,CH3CO2H
E)Two of these choices.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
The product of the following reaction sequence may be described as? 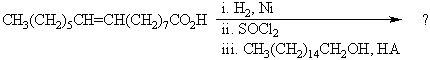
A)Alkoxyalkane
B)Alkyl alkanoate
C)Alkyl alkenoate
D)Acyl glycerol
E)Acyl Alkane
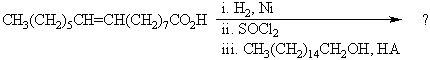
A)Alkoxyalkane
B)Alkyl alkanoate
C)Alkyl alkenoate
D)Acyl glycerol
E)Acyl Alkane

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following could be used to prepare myristic acid,CH3(CH2)12COOH?
A)CH3(CH2)11CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)11CH2Br,Mg,(C2H5)2O; then CO2; then H3O+
C)CH3(CH2)12CHO,Ag2O,OH-; then H3O+
D)Two of these choices.
E)All of these choices.
A)CH3(CH2)11CH2Br,CN-,heat; then H3O+,heat
B)CH3(CH2)11CH2Br,Mg,(C2H5)2O; then CO2; then H3O+
C)CH3(CH2)12CHO,Ag2O,OH-; then H3O+
D)Two of these choices.
E)All of these choices.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
Which of these reagents would not react with oleic acid?
A)H2,Ni
B)PBr3
C)CH3MgI
D)NH3/H2O
E)NaBH4
A)H2,Ni
B)PBr3
C)CH3MgI
D)NH3/H2O
E)NaBH4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the repeating unit of natural rubber? 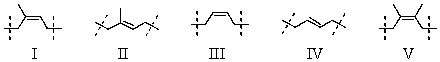
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
What product would you expect when progesterone is treated with one molar equivalent of hydrogen in the presence of a platinum catalyst? 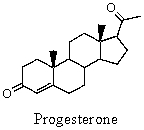
A)5 -Pregnane-3,20-dione
B)5 -Pregnane-3,20-dione
C)5 -Estrane-3,20-dione
D)5 -Estrane-3,20-dione
E)5 -Androstane-3,20-dione

A)5 -Pregnane-3,20-dione
B)5 -Pregnane-3,20-dione
C)5 -Estrane-3,20-dione
D)5 -Estrane-3,20-dione
E)5 -Androstane-3,20-dione

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Which of these is a correct systematic name for progesterone? 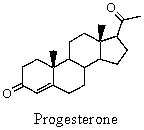
A)2-Estrene-4,20-dione
B)5-Androstene-4,19-dione
C)4-Pregnene-3,20-dione
D)5-Cholestene-5,19-dione
E)4-Cholene-3,20-dione

A)2-Estrene-4,20-dione
B)5-Androstene-4,19-dione
C)4-Pregnene-3,20-dione
D)5-Cholestene-5,19-dione
E)4-Cholene-3,20-dione

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
The synthesis of cortisone required placing a ketone function at the 11-position of a steroid.Where is position 11? 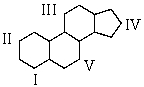
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
Which compound is a sesquiterpene? 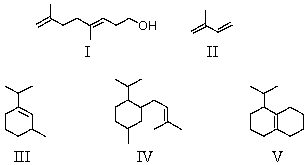
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
The reaction of cholesterol with dilute aqueous KMnO4 at 0-5 °C produces which of these compounds (A and B rings only shown)? 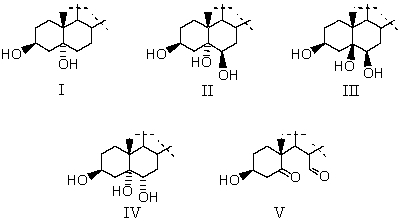
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
Essential oils are constituted by
A)fatty acids and glycerol
B)triacylglycerol
C)terpenes only
D)glycerol
E)terpenoids and terpenes
A)fatty acids and glycerol
B)triacylglycerol
C)terpenes only
D)glycerol
E)terpenoids and terpenes

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these is a male sex hormone?
A)Estrone
B)Cholic acid
C)Testosterone
D)Cortisone
E)Estradiol
A)Estrone
B)Cholic acid
C)Testosterone
D)Cortisone
E)Estradiol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
In -carotene,how many tail-to-tail links of isoprene units are there? 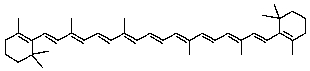
A)1
B)2
C)3
D)4
E)More than 4

A)1
B)2
C)3
D)4
E)More than 4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
In the vulcanization of rubber,
A)natural rubber is heated with sulfur.
B)reaction occurs at allylic positions.
C)cross-linking results in a hardening of the rubber.
D)disulfide bridges are formed.
E)All of these choices.
A)natural rubber is heated with sulfur.
B)reaction occurs at allylic positions.
C)cross-linking results in a hardening of the rubber.
D)disulfide bridges are formed.
E)All of these choices.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
Which is the proper representation of three successive isoprene units in natural rubber? 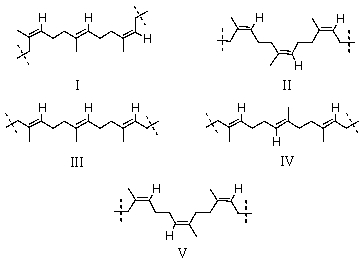
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
Which is an untrue statement concerning cholesterol?
A)Cholesterol decolorizes a solution of Br2 in CCl4.
B)Cholesterol reacts with 2,4-dinitrophenylhydrazine.
C)Cholesterol is optically active.
D)Cholesterol is water-insoluble.
E)Cholesterol reacts with H2/Pd
A)Cholesterol decolorizes a solution of Br2 in CCl4.
B)Cholesterol reacts with 2,4-dinitrophenylhydrazine.
C)Cholesterol is optically active.
D)Cholesterol is water-insoluble.
E)Cholesterol reacts with H2/Pd

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
Which reagent might be used to convert 5 -cholest-1-en-3-ol into 5 -cholestan-3-ol?
A)CrO3/pyridine
B)KMnO4/H2O
C)CH3MgI
D)H2/Pt
E)Li/C2H5NH2
A)CrO3/pyridine
B)KMnO4/H2O
C)CH3MgI
D)H2/Pt
E)Li/C2H5NH2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
To which class of terpenes does the terpene shown below,bisabolene,belong? 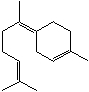
A)Monoterpenes
B)Sesquiterpenes
C)Diterpenes
D)Triterpenes
E)Tetraterpenes

A)Monoterpenes
B)Sesquiterpenes
C)Diterpenes
D)Triterpenes
E)Tetraterpenes

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is a female sex hormone?
A)Ergosterol
B)Estradiol
C)Cortisone
D)Androsterone
E)Cholic acid
A)Ergosterol
B)Estradiol
C)Cortisone
D)Androsterone
E)Cholic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
How many isoprene units are in vitamin A? 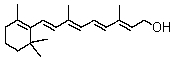
A)1
B)2
C)3
D)4
E)More than 4

A)1
B)2
C)3
D)4
E)More than 4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
Which reagent might serve as the basis for a simple chemical test that would distinguish between 5 -cholest-1-en-3-one and 5 -cholestan-3-one?
A)Ag(NH3)2+
B)CrO3/H2SO4
C)Br2/CCl4
D)NaOH/H2O
E)C6H5NHNH2
A)Ag(NH3)2+
B)CrO3/H2SO4
C)Br2/CCl4
D)NaOH/H2O
E)C6H5NHNH2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
What product would be obtained by catalytic hydrogenation of 5-cholesten-3 -ol?
A)5 -Cholestan-3 -ol
B)5 -Cholestan-3 -ol.
C)5 -Cholestan-3 -ol
D)5 -Cholestan-3 -ol
E)5-Cholesten-3 ,6 -diol
A)5 -Cholestan-3 -ol
B)5 -Cholestan-3 -ol.
C)5 -Cholestan-3 -ol
D)5 -Cholestan-3 -ol
E)5-Cholesten-3 ,6 -diol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements regarding lipids is not true?
A)Lipids are soluble in non-polar organic solvents.
B)All lipids have the same functional groups.
C)Lipids include waxes,steroids,and triacylglycerols.
D)Lipids have little in common except their solubility.
E)Many lipids have biological roles.
A)Lipids are soluble in non-polar organic solvents.
B)All lipids have the same functional groups.
C)Lipids include waxes,steroids,and triacylglycerols.
D)Lipids have little in common except their solubility.
E)Many lipids have biological roles.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
Which is the correct systematic name for the steroid shown below? 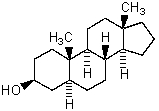
A)5 -Androstan-3 -ol
B)5 -Androstan-3 -ol
C)5 -Androstan-3 -ol
D)5 -Androstan-3 -ol
E)5 -Estan-3 -ol

A)5 -Androstan-3 -ol
B)5 -Androstan-3 -ol
C)5 -Androstan-3 -ol
D)5 -Androstan-3 -ol
E)5 -Estan-3 -ol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
The compounds I and II are: 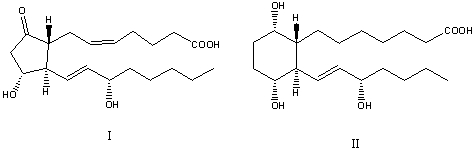
A)Prostaglandins.
B)Terpenoids.
C)I is a prostaglandin; II is not a prostaglandin.
D)I is an E type prostaglandin; II is an F type prostaglandin.
E)Both are fatty acids.

A)Prostaglandins.
B)Terpenoids.
C)I is a prostaglandin; II is not a prostaglandin.
D)I is an E type prostaglandin; II is an F type prostaglandin.
E)Both are fatty acids.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
Which of these lipids yields glycerol upon hydrolysis?
A)A lecithin
B)A phosphatidylserine
C)A cephalin
D)A triacylglycerol
E)All of these choices.
A)A lecithin
B)A phosphatidylserine
C)A cephalin
D)A triacylglycerol
E)All of these choices.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a phosphatidic acid? 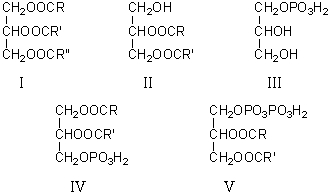
A)I
B)II
C)III
D)IV
E)V
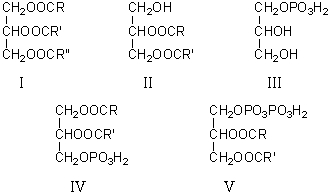
A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
Which type of phosphatide has the structural unit: 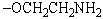
A)Cephalins
B)Phosphatidyl serines
C)Plasmalogens
D)Lecithins
E)Both cephalins and plasmalogens
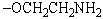
A)Cephalins
B)Phosphatidyl serines
C)Plasmalogens
D)Lecithins
E)Both cephalins and plasmalogens

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
How many stereogenic centers are there in cholesterol? 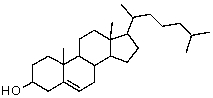
A)2
B)4
C)6
D)8
E)16

A)2
B)4
C)6
D)8
E)16

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
Some prostaglandins induce inflammation.Aspirin is an anti-inflammatory drug because:
A)Apparently it acetylates the enzyme cyclooxygenase.
B)It reacts with arachidonic acid preventing the synthesis of inflammatory prostaglandins.
C)It blocks the synthesis of the catalytic enzyme leading to inflammatory prostaglandins.
D)It reacts with inflammatory prostaglandins preventing them for being active.
E)It renders the arachidonic acid inactive in the synthesis of prostaglandins.
A)Apparently it acetylates the enzyme cyclooxygenase.
B)It reacts with arachidonic acid preventing the synthesis of inflammatory prostaglandins.
C)It blocks the synthesis of the catalytic enzyme leading to inflammatory prostaglandins.
D)It reacts with inflammatory prostaglandins preventing them for being active.
E)It renders the arachidonic acid inactive in the synthesis of prostaglandins.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
Which of these lipids does not yield glycerol upon hydrolysis?
A)A lecithin
B)A sphingolipid
C)A cephalin
D)A triacylglycerol
E)A plasmalogen
A)A lecithin
B)A sphingolipid
C)A cephalin
D)A triacylglycerol
E)A plasmalogen

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
Which type of phosphatide contains the structural unit shown below? 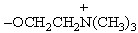
A)Cephalins
B)Phosphatidyl serines
C)Plasmalogens
D)Lecithins
E)Both plasmalogens and lecithins
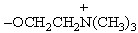
A)Cephalins
B)Phosphatidyl serines
C)Plasmalogens
D)Lecithins
E)Both plasmalogens and lecithins

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
The biosynthesis of one series of prostaglandins begins with which of these fatty acids?
A)Palmitic acid
B)Stearic acid
C)Oleic acid
D)Linoleic acid
E)Arachidonic acid
A)Palmitic acid
B)Stearic acid
C)Oleic acid
D)Linoleic acid
E)Arachidonic acid

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
Which of these lipids yields choline upon hydrolysis?
A)A lecithin only
B)A sphingomyelin and a cerebroside
C)A cephalin and a lecithin
D)A cerebroside and a cephalin
E)A lecithin and a sphingomyelin
A)A lecithin only
B)A sphingomyelin and a cerebroside
C)A cephalin and a lecithin
D)A cerebroside and a cephalin
E)A lecithin and a sphingomyelin

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
Choline cannot be found as a product of hydrolysis of any representative of this class of lipids.
A)Cerebrosides
B)Phosphatidylserine
C)Plasmalogens
D)Waxes
E)None of these choices yield choline upon hydrolysis
A)Cerebrosides
B)Phosphatidylserine
C)Plasmalogens
D)Waxes
E)None of these choices yield choline upon hydrolysis

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
What of the following is a (are)correct structure(s)for prostaglandin(s)? 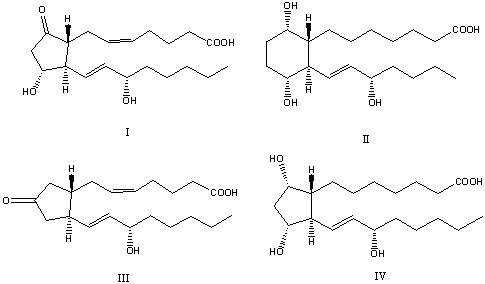
A)I and II
B)I,III and IV
C)III
D)II
E)I and IV

A)I and II
B)I,III and IV
C)III
D)II
E)I and IV

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
Which of these is a wax?
A)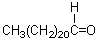
B)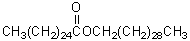
C)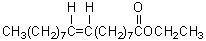
D)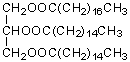
E)CH3(CH2)24CH2OH
A)
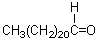
B)
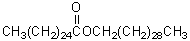
C)
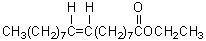
D)
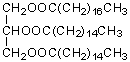
E)CH3(CH2)24CH2OH

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
Which type of lipid gives the following products on saponification? HOCH2CHOHCH2OH RCO2¯ R'CO2¯ PO43¯ [HOCH2CH2N(CH3)3]+OH-
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
Which type of lipid gives these products on saponification? HOCH2CHOHCH2OH RCO2¯ RCH2CHO PO43¯ HOCH2CH2NH2
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
Estrone can be separated from testosterone by
A)Treatment of the mixture with aqueous NaOH and extraction with ethyl ether.Estrone is extracted in the ether phase.
B)Treatment of the mixture with NaOH and extraction with ethyl ether.Testosterone is extracted in the ether phase.
C)Treatment of the mixture with NaOH and extraction with ethyl ether.Estrone and testosterone are extracted in the ether phase.
D)Treatment of the mixture with NaHCO3 and extraction with ethyl ether.Testosterone is extracted in the ether phase.
E)Treatment of the mixture with NaHCO3 and extraction with ethyl ether.Estrone is extracted in the ether phase.
A)Treatment of the mixture with aqueous NaOH and extraction with ethyl ether.Estrone is extracted in the ether phase.
B)Treatment of the mixture with NaOH and extraction with ethyl ether.Testosterone is extracted in the ether phase.
C)Treatment of the mixture with NaOH and extraction with ethyl ether.Estrone and testosterone are extracted in the ether phase.
D)Treatment of the mixture with NaHCO3 and extraction with ethyl ether.Testosterone is extracted in the ether phase.
E)Treatment of the mixture with NaHCO3 and extraction with ethyl ether.Estrone is extracted in the ether phase.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
d-Galactose is an example of a class of compounds that can be formed during the hydrolysis of:
A)Sphingomyelins
B)Cerebrosides
C)Plasmalogens
D)Waxes
E)Both plasmalogens and waxes
A)Sphingomyelins
B)Cerebrosides
C)Plasmalogens
D)Waxes
E)Both plasmalogens and waxes

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
Shown below is the formula for the antiinflammatory drug called prednisone.What is a correct systematic name for prednisone? 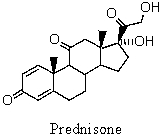
A)17 ,21-Dihydroxypregna-1,4-diene-3,11,20-trione
B)17 ,21-Dihydroxypregna-1,4-diene-3,11,20-trione
C)17 ,19-Dihydroxypregna-1,4-diene-3,11,20-trione
D)17 ,19-Dihydroxypregna-1,4-diene-3,11,20-trione
E)17 ,21-Dihydroandrostan-1,4-diene-3,11,20-trione

A)17 ,21-Dihydroxypregna-1,4-diene-3,11,20-trione
B)17 ,21-Dihydroxypregna-1,4-diene-3,11,20-trione
C)17 ,19-Dihydroxypregna-1,4-diene-3,11,20-trione
D)17 ,19-Dihydroxypregna-1,4-diene-3,11,20-trione
E)17 ,21-Dihydroandrostan-1,4-diene-3,11,20-trione

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Which type of lipid gives these products on saponification? HOCH2CHOHCH2OH RCO2¯ R'CO2¯ PO43¯ HOCH2CH2NH2
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen
A)Fat
B)Wax
C)Lecithin
D)Cephalin
E)Plasmalogen

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following characteristics are found in the class of C20 carboxylic acids called prostaglandins?
A)A five membered ring
B)One or more double bonds
C)Several oxygen containing groups
D)Two of the above
E)All of these choices
A)A five membered ring
B)One or more double bonds
C)Several oxygen containing groups
D)Two of the above
E)All of these choices

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


