Deck 14: Carboxylic Acids, esters, amines, and Amides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/103
Play
Full screen (f)
Deck 14: Carboxylic Acids, esters, amines, and Amides
1
The common name of the compound 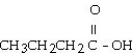 is ________.
is ________.
A)acetic acid
B)propanoic acid
C)propionic acid
D)butanoic acid
E)butyric acid
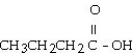 is ________.
is ________.A)acetic acid
B)propanoic acid
C)propionic acid
D)butanoic acid
E)butyric acid
butyric acid
2
Which functional group is found in a carboxylic acid?
A)-OH
B) O
-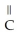 -OH
-OH
C) O
-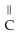 - O -
- O -
D) OH
|
- C - OH
|
E)-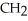 -OH
-OH
A)-OH
B) O
-
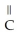 -OH
-OHC) O
-
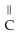 - O -
- O -D) OH
|
- C - OH
|
E)-
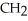 -OH
-OHO
-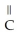 -OH
-OH
-
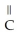 -OH
-OH 3
The neutralization of formic acid by NaOH produces ________.
A)sodium formate as the only product
B)formate ion and hydronium ion
C)sodium formaldehyde
D)methyl alcohol
E)sodium formate and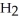 O
O
A)sodium formate as the only product
B)formate ion and hydronium ion
C)sodium formaldehyde
D)methyl alcohol
E)sodium formate and
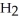 O
Osodium formate and 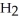 O
O
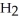 O
O 4
What happens to water solubility as the hydrocarbon chain length increases in carboxylic acids?
A)It increases.
B)It decreases.
C)It stays the same.
A)It increases.
B)It decreases.
C)It stays the same.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
5
What is the common name for ethanoic acid?
A)butyric acid
B)formic acid
C)citric acid
D)stearic acid
E)acetic acid
A)butyric acid
B)formic acid
C)citric acid
D)stearic acid
E)acetic acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is most soluble in water?
A)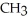 -
- 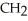
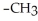
B)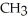
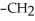
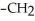 -O
-O 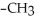
C)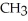
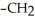
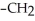
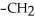 -OH
-OH
D)O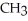 -
- 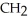 -
- 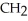
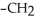 -
- 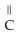 -OH
-OH
E)O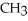 -
- 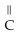 -OH
-OH
A)
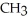 -
- 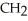
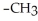
B)
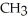
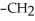
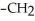 -O
-O 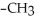
C)
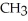
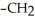
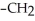
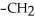 -OH
-OHD)O
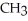 -
- 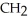 -
- 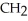
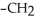 -
- 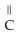 -OH
-OHE)O
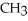 -
- 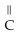 -OH
-OH
Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
7
What is the irritating acid found in ant and bee stings?
A)acetic acid
B)formic acid
C)citric acid
D)butyric acid
E)stearic acid
A)acetic acid
B)formic acid
C)citric acid
D)butyric acid
E)stearic acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
8
Many of the fragrances of flowers and the flavors of fruits are due to ________.
A)ethers
B)carboxylic acids
C)esters
D)amines
E)amides
A)ethers
B)carboxylic acids
C)esters
D)amines
E)amides

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
9
Which carboxylic acid in the list below is an aromatic carboxylic acid?
A)acetic acid
B)benzoic acid
C)butyric acid
D)benzene
E)citric acid
A)acetic acid
B)benzoic acid
C)butyric acid
D)benzene
E)citric acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
10
In water solution,how does dilute acetic acid behave?
A)as a strong acid
B)as a weak acid
C)as a strong base
D)as a weak base
E)as a neutral compound
A)as a strong acid
B)as a weak acid
C)as a strong base
D)as a weak base
E)as a neutral compound

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
11
When compared to sulfuric acid,how strong are carboxylic acids?
A)stronger
B)just as strong
C)weaker
D)not acidic at all
A)stronger
B)just as strong
C)weaker
D)not acidic at all

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
12
The functional group in acetic acid is called the ________.
A)hydroxyl group
B)aldehyde group
C)carbonyl group
D)carboxyl group
E)ester group
A)hydroxyl group
B)aldehyde group
C)carbonyl group
D)carboxyl group
E)ester group

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
13
The IUPAC name of this compound is ________. 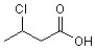
A)3-chloro-1-butanol
B)chlorobutyric acid
C)3-chlorobutyric acid
D)2-chlorobutanoic acid
E)3-chlorobutanoic acid

A)3-chloro-1-butanol
B)chlorobutyric acid
C)3-chlorobutyric acid
D)2-chlorobutanoic acid
E)3-chlorobutanoic acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound below contains an ester functional group?
A)OH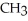 -
- 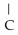 H-
H- 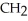 -
- 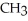
B)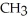 -
- 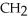 -O-
-O- 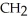 -
- 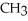
C)O H-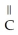 -O-
-O- 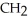 -
- 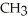
D)O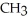 -
- 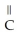 -
- 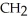 -
- 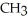
E)O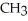 -
- 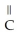 -OH
-OH
A)OH
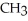 -
- 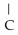 H-
H- 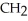 -
- 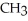
B)
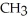 -
- 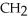 -O-
-O- 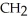 -
- 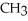
C)O H-
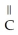 -O-
-O- 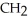 -
- 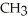
D)O
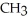 -
- 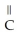 -
- 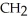 -
- 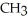
E)O
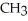 -
- 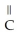 -OH
-OH
Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
15
A carboxylic acid is named in the IUPAC system by replacing the e in the name of the parent alkane with ________.
A)oic acid
B)oic
C)carboxylic acid
D)acid
E)oate
A)oic acid
B)oic
C)carboxylic acid
D)acid
E)oate

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
16
This functional group is known as a(n)________. O  - C - O -C
- C - O -C
A)ester
B)carboxylic acid
C)alcohol
D)aldehyde
E)acetal
 - C - O -C
- C - O -CA)ester
B)carboxylic acid
C)alcohol
D)aldehyde
E)acetal

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
17
What kind of taste do carboxylic acids have?
A)sweet
B)sour
C)fruity
D)slippery
E)oily
A)sweet
B)sour
C)fruity
D)slippery
E)oily

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is found in vinegar?
A)nitric acid
B)formic acid
C)acetic acid
D)propionic acid
E)butyric acid
A)nitric acid
B)formic acid
C)acetic acid
D)propionic acid
E)butyric acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these functional groups is likely to give a sour taste to a food?
A)ester
B)ether
C)ketone
D)carboxylic acid
E)thiol
A)ester
B)ether
C)ketone
D)carboxylic acid
E)thiol

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
20
What is the common name of this compound? O 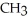 -
- 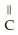 -O -
-O - 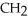 -
- 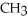
A)ethyl methyl ester
B)diethyl ester
C)ethyl methanoate
D)2-ether-2-butanone
E)ethyl acetate
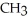 -
- 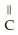 -O -
-O - 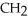 -
- 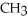
A)ethyl methyl ester
B)diethyl ester
C)ethyl methanoate
D)2-ether-2-butanone
E)ethyl acetate

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
21
The first word in the name of an ester is derived from the ________ used in the esterification.
A)carboxylic acid
B)alcohol
C)ether
D)ester
E)amide
A)carboxylic acid
B)alcohol
C)ether
D)ester
E)amide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
22
The alcohol and carboxylic acid required to form propyl ethanoate are ________.
A)methanol and propionic acid
B)ethanol and propionic acid
C)propanol and propanoic acid
D)1-propanol and ethanoic acid
E)2-propanol and ethanoic acid
A)methanol and propionic acid
B)ethanol and propionic acid
C)propanol and propanoic acid
D)1-propanol and ethanoic acid
E)2-propanol and ethanoic acid

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
23
What chemical process is responsible for the smell of vinegar in an old bottle of aspirin?
A)reduction
B)hydration
C)hydrolysis
D)esterification
E)dissolution
A)reduction
B)hydration
C)hydrolysis
D)esterification
E)dissolution

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is the reaction for the saponification of methyl acetate?
A)O O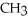 -
- 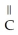 -O-
-O- 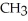 + NaOH →
+ NaOH → 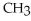 -
- 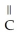 -OH +
-OH + 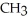 -
- 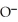
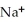
B)O O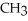 -
- 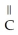 -O-
-O- 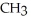 + NaOH →
+ NaOH → 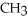 -
- 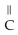 -
- 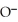
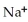 +
+ 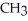 -OH
-OH
C)O O H-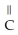 -O-
-O- 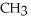 +
+ 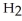 O → H-
O → H- 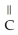 -
- 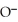 +
+ 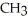 -
- 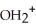
D)O O H-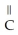 -O-
-O- 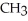 + NaOH → H-
+ NaOH → H- 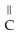 -
- 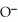
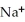 +
+ 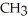 -OH
-OH
E)O O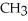 -
- 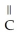 -O-
-O- 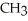 +
+ 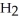 O →
O → 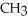 -
- 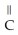 -
- 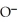 +
+ 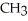 -
- 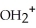
A)O O
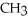 -
- 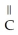 -O-
-O- 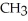 + NaOH →
+ NaOH → 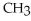 -
- 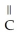 -OH +
-OH + 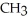 -
- 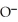
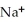
B)O O
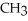 -
- 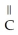 -O-
-O- 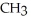 + NaOH →
+ NaOH → 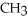 -
- 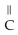 -
- 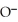
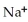 +
+ 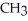 -OH
-OHC)O O H-
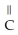 -O-
-O- 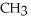 +
+ 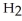 O → H-
O → H- 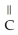 -
- 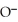 +
+ 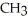 -
- 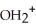
D)O O H-
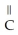 -O-
-O- 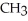 + NaOH → H-
+ NaOH → H- 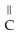 -
- 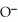
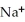 +
+ 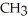 -OH
-OHE)O O
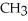 -
- 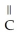 -O-
-O- 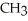 +
+ 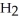 O →
O → 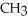 -
- 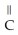 -
- 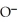 +
+ 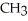 -
- 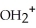

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following represents the complete neutralization of dimethylamine?
A)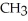 -NH +
-NH + 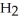 O →
O → 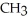 -
- 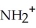 +
+ 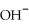 | |
| | 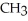
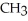
B)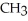 -NH + NaOH →
-NH + NaOH → 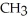 -N-
-N- 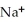 +
+ 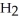 O | |
O | | 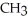
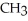
C)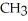 -NH + HCl →
-NH + HCl → 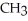 -
- 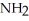 +
+ 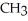 Cl |
Cl | 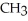
D)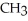 -NH +
-NH + 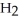 O →
O → 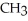 -OH +
-OH + 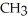 -
- 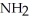 |
| 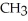
E)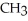 NH + HCl →
NH + HCl → 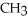
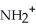 + Cl- | |
+ Cl- | | 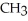
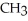
A)
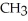 -NH +
-NH + 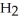 O →
O → 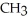 -
- 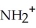 +
+ 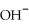 | |
| | 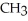
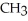
B)
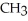 -NH + NaOH →
-NH + NaOH → 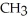 -N-
-N- 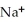 +
+ 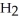 O | |
O | | 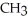
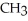
C)
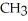 -NH + HCl →
-NH + HCl → 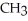 -
- 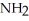 +
+ 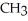 Cl |
Cl | 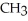
D)
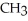 -NH +
-NH + 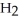 O →
O → 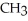 -OH +
-OH + 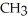 -
- 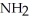 |
| 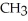
E)
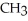 NH + HCl →
NH + HCl → 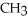
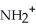 + Cl- | |
+ Cl- | | 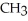
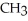

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
26
Diethylamine and HCl react to produce ________.
A)diethyl chloride
B)diethylammonium chloride
C)ethylammonium chloride
D)ammonium chloride
E)butylammonium chloride
A)diethyl chloride
B)diethylammonium chloride
C)ethylammonium chloride
D)ammonium chloride
E)butylammonium chloride

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
27
The common name of this compound 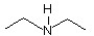 is ________.
is ________.
A)ethylamine
B)ethylethylamine
C)diethylamine
D)dimethylamine
E)ethylmethylamine
 is ________.
is ________.A)ethylamine
B)ethylethylamine
C)diethylamine
D)dimethylamine
E)ethylmethylamine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
28
The reactants that will form an ester in the presence of an acid catalyst are ________.
A)two carboxylic acids
B)two alcohols
C)a carboxylic acid and an alcohol
D)an aldehyde and an alcohol
E)two aldehydes
A)two carboxylic acids
B)two alcohols
C)a carboxylic acid and an alcohol
D)an aldehyde and an alcohol
E)two aldehydes

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
29
When this compound undergoes hydrolysis in acid,what product(s)are obtained? 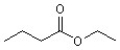
A)butanoic acid and ethanol
B)butanoic acid
C)ethanol
D)ethanoic acid and butanol
E)butanoic acid and butanol

A)butanoic acid and ethanol
B)butanoic acid
C)ethanol
D)ethanoic acid and butanol
E)butanoic acid and butanol

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
30
Which of these compounds is the ester formed from the reaction of acetic acid and 1-propanol?
A)O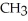 -
- 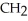 -
- 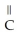 -O-
-O- 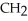 -
- 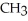
B)OH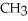 -
- 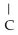 -OH
-OH 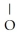 -
- 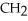 -
- 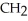 -
- 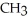
C)OH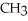
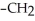 -
- 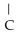 -OH
-OH 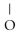 -
- 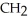 -
- 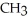
D)O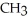 -
- 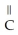 -O-
-O- 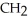 -
- 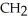 -
- 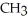
E)O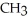 -
- 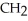 -
- 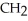 -O-
-O- 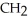 -
- 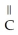 -OH
-OH
A)O
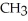 -
- 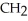 -
- 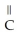 -O-
-O- 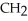 -
- 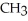
B)OH
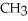 -
- 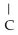 -OH
-OH 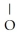 -
- 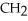 -
- 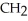 -
- 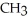
C)OH
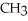
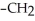 -
- 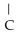 -OH
-OH 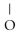 -
- 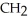 -
- 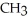
D)O
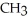 -
- 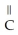 -O-
-O- 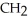 -
- 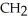 -
- 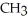
E)O
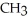 -
- 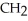 -
- 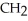 -O-
-O- 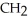 -
- 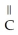 -OH
-OH
Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
31
The compound 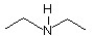 is classified as a ________.
is classified as a ________.
A)primary amine
B)secondary amine
C)tertiary amine
D)quaternary amine
E)hydrated amine
 is classified as a ________.
is classified as a ________.A)primary amine
B)secondary amine
C)tertiary amine
D)quaternary amine
E)hydrated amine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
32
What is the product of the reaction of an alcohol and a carboxylic acid when heated together under acidic conditions?
A)an ether
B)an ester
C)a salt
D)a ketone
E)an aldehyde
A)an ether
B)an ester
C)a salt
D)a ketone
E)an aldehyde

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
33
What kind of conditions can produce hydrolysis of an ester?
A)acidic
B)basic
C)either acidic or basic
D)neither acidic nor basic
A)acidic
B)basic
C)either acidic or basic
D)neither acidic nor basic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds cannot form hydrogen bonds with water?
A)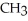
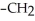
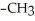
B)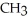
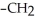 -OH
-OH
C)O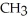 -
- 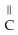 -OH
-OH
D)O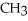
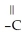 -O-
-O- 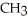
E)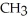 -
- 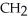

A)
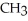
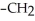
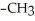
B)
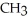
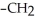 -OH
-OHC)O
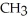 -
- 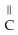 -OH
-OHD)O
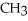
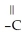 -O-
-O- 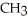
E)
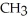 -
- 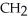


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
35
The compound 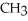
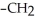
 is classified as a ________.
is classified as a ________.
A)primary amine
B)secondary amine
C)tertiary amine
D)quaternary amine
E)hydrated amine
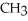
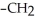
 is classified as a ________.
is classified as a ________.A)primary amine
B)secondary amine
C)tertiary amine
D)quaternary amine
E)hydrated amine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
36
When ethylamine dissolves in water,a solution of ________ is produced.
A)ammonia
B)ethylammonium and hydroxide
C)ethylamine
D)ethylhydroxide
E)ethylhydroxylate
A)ammonia
B)ethylammonium and hydroxide
C)ethylamine
D)ethylhydroxide
E)ethylhydroxylate

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
37
What is the common name of this compound? 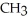 -N-
-N- 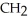 -
- 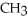
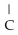
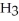
A)trimethylamine
B)diethylamine
C)ethylmethylamine
D)ethylmethylnitride
E)ethyldimethylamine
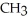 -N-
-N- 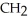 -
- 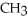
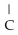
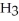
A)trimethylamine
B)diethylamine
C)ethylmethylamine
D)ethylmethylnitride
E)ethyldimethylamine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
38
The splitting apart of an ester in the presence of a strong acid and water is called ________.
A)hydrolysis
B)saponification
C)neutralization
D)esterification
E)reduction
A)hydrolysis
B)saponification
C)neutralization
D)esterification
E)reduction

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the reaction for the acid hydrolysis of ethyl formate?
A)O O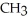 -
- 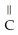 -O-
-O- 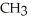 + NaOH →
+ NaOH → 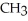 -
- 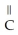 -
- 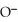
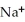 +
+ 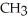 -OH
-OH
B)O O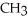 -
- 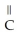 -O-
-O- 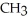 +
+ 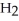 O →
O → 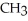 -
- 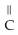 -OH +
-OH + 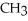 -OH
-OH
C)O O H-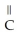 -O-
-O- 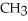 +
+ 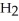 O → H-
O → H- 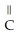 -OH +
-OH + 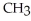 -OH
-OH
D)O O H-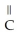 -O-
-O- 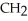 -
- 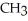 +
+ 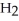 O → H-
O → H- 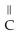 -OH +
-OH + 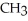 -
- 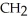 -OH
-OH
E)O OH H-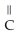 -O-
-O- 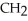 -
- 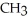 +
+ 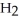 O → H-
O → H- 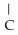 -O-
-O- 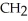 -
- 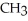
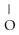 H
H
A)O O
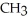 -
- 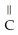 -O-
-O- 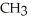 + NaOH →
+ NaOH → 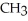 -
- 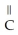 -
- 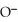
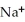 +
+ 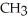 -OH
-OHB)O O
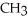 -
- 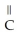 -O-
-O- 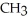 +
+ 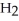 O →
O → 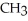 -
- 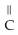 -OH +
-OH + 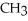 -OH
-OHC)O O H-
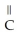 -O-
-O- 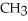 +
+ 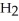 O → H-
O → H- 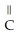 -OH +
-OH + 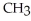 -OH
-OHD)O O H-
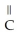 -O-
-O- 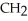 -
- 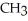 +
+ 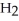 O → H-
O → H- 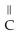 -OH +
-OH + 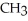 -
- 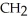 -OH
-OHE)O OH H-
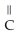 -O-
-O- 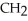 -
- 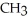 +
+ 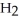 O → H-
O → H- 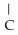 -O-
-O- 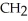 -
- 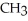
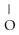 H
H
Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction of an ester with NaOH is known as ________.
A)esterification
B)neutralization
C)saponification
D)reduction
E)oxidation
A)esterification
B)neutralization
C)saponification
D)reduction
E)oxidation

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
41
The odor of an amine can be neutralized with ________.
A)water
B)acids
C)bases
D)detergents
E)solvents
A)water
B)acids
C)bases
D)detergents
E)solvents

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
42
How many alkyl substituents does N-ethyl-N-methylaniline have?
A)one
B)two
C)three
D)eight
E)none
A)one
B)two
C)three
D)eight
E)none

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
43
Amines contain the element ________.
A)nitrogen
B)oxygen
C)sulfur
D)astatine
E)arginine
A)nitrogen
B)oxygen
C)sulfur
D)astatine
E)arginine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
44
In what form are amine-containing drugs often administered?
A)free base
B)sodium salt
C)ammonium salt
D)water solution
E)oil solution
A)free base
B)sodium salt
C)ammonium salt
D)water solution
E)oil solution

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
45
With the correct choice of acid,acid hydrolysis of acetamide could produce ________.
A)acetic acid and ammonium chloride
B)acetic acid and methylamine
C)ethanol and ammonia
D)acetaldehyde and ammonium hydroxide
E)formic acid and ethylamine
A)acetic acid and ammonium chloride
B)acetic acid and methylamine
C)ethanol and ammonia
D)acetaldehyde and ammonium hydroxide
E)formic acid and ethylamine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
46
When acetic acid reacts with ammonia, 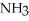 ,the reaction yields ________.
,the reaction yields ________.
A)acetamine
B)ammonium acetate
C)ethylammonium hydroxide
D)amino acetate
E)acetamide
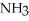 ,the reaction yields ________.
,the reaction yields ________.A)acetamine
B)ammonium acetate
C)ethylammonium hydroxide
D)amino acetate
E)acetamide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
47
In what kind of amine is the nitrogen atom bonded to two carbon atoms?
A)primary
B)secondary
C)tertiary
D)quaternary
E)amide
A)primary
B)secondary
C)tertiary
D)quaternary
E)amide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
48
What is the functional group in the following compound? O 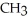 -
- 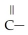 NH
NH 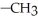
A)ketone
B)carboxylic acid
C)ester
D)amine
E)amide
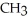 -
- 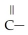 NH
NH 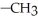
A)ketone
B)carboxylic acid
C)ester
D)amine
E)amide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
49
Physiologically active nitrogen-containing compounds produced by plants are called ________.
A)aromatics
B)alkaloids
C)esters
D)polymers
E)ethers
A)aromatics
B)alkaloids
C)esters
D)polymers
E)ethers

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
50
Amines are ________.
A)Brønsted-Lowry bases
B)Brønsted-Lowry acids
C)neutral in water solution
D)unreactive
A)Brønsted-Lowry bases
B)Brønsted-Lowry acids
C)neutral in water solution
D)unreactive

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
51
What functional group is always found in alkaloids (such as caffeine,nicotine,and digitalis)?
A)amide
B)acid
C)ether
D)amine
E)ester
A)amide
B)acid
C)ether
D)amine
E)ester

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
52
What are alkaloids?
A)physiologically active nitrogen compounds derived from plants
B)anesthetics found in plants
C)flavoring agents found in fruits and vegetables
D)preservatives found in animal tissue
E)natural steroids
A)physiologically active nitrogen compounds derived from plants
B)anesthetics found in plants
C)flavoring agents found in fruits and vegetables
D)preservatives found in animal tissue
E)natural steroids

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
53
What physiologically active amine is responsible for the signs and symptoms encountered in an allergic reaction?
A)histamine
B)epinephrine
C)diphenhydramine
D)phenylephrine
E)dopamine
A)histamine
B)epinephrine
C)diphenhydramine
D)phenylephrine
E)dopamine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
54
Amines can form ________ bonds with water molecules.
A)oxygen
B)hydrogen
C)nonpolar
D)metallic
E)triple
A)oxygen
B)hydrogen
C)nonpolar
D)metallic
E)triple

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
55
Nicotine,coniine,quinine,atropine,and morphine are all examples of ________.
A)ethers
B)esters
C)carboxylic acids
D)alkaloids
E)amides
A)ethers
B)esters
C)carboxylic acids
D)alkaloids
E)amides

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
56
Aniline is a(n)________.
A)primary aromatic amine
B)secondary aromatic amine
C)heterocyclic amine
D)aliphatic amine
E)tertiary amine
A)primary aromatic amine
B)secondary aromatic amine
C)heterocyclic amine
D)aliphatic amine
E)tertiary amine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
57
The amide formed in the reaction of benzoic acid and ethylamine is ________.
A)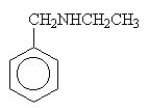
B)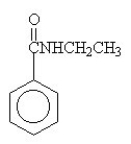
C)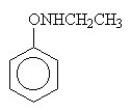
D)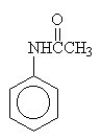
E)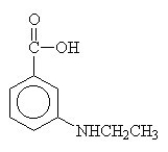
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
58
The prefix nor- in a drug name means that there is ________.
A)one more amine in the new molecule than in the original
B)one more methyl group on the nitrogen atom in the new molecule than in the original
C)one less methyl group on the nitrogen atom in the new molecule than in the original
D)one less amine in the new molecule than in the original
E)one less double bond in the new molecule than in the original
A)one more amine in the new molecule than in the original
B)one more methyl group on the nitrogen atom in the new molecule than in the original
C)one less methyl group on the nitrogen atom in the new molecule than in the original
D)one less amine in the new molecule than in the original
E)one less double bond in the new molecule than in the original

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
59
A deficiency of which amine is responsible for the signs and symptoms of Parkinson's disease?
A)histamine
B)dopamine
C)epinephrine
D)diphenhydramine
E)methedrine
A)histamine
B)dopamine
C)epinephrine
D)diphenhydramine
E)methedrine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
60
What prefix is used to show that a small alkyl group is attached to the nitrogen of aniline and not to the aromatic ring?
A)N-
B)C-
C)Roman numerals
D)Greek letters
E)iso-
A)N-
B)C-
C)Roman numerals
D)Greek letters
E)iso-

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
61
Most of the carboxylic acid in an aqueous solution is ionized.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
62
Amides are derivatives of ________ and ________.
A)amines,esters
B)amines,carboxylic acids
C)alkanes,amines
D)carboxylic acids,alcohols
E)alcohols,carboxylic acids
A)amines,esters
B)amines,carboxylic acids
C)alkanes,amines
D)carboxylic acids,alcohols
E)alcohols,carboxylic acids

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
63
What kind of compound is urea?
A)ester
B)acid
C)amide
D)ketone
E)amine
A)ester
B)acid
C)amide
D)ketone
E)amine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
64
The IUPAC name of this compound is ________. 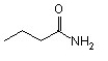
A)pentanamide
B)butyramide
C)butylamine
D)butanamide
E)propanamide

A)pentanamide
B)butyramide
C)butylamine
D)butanamide
E)propanamide

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
65
Butyl alcohol is one of the reactants used to make methyl butyrate.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
66
To which chemical class does phenobarbital belong?
A)ester
B)amine
C)amide
D)alkane
E)ether
A)ester
B)amine
C)amide
D)alkane
E)ether

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
67
Amides having fewer than ________ carbons are generally water soluble.
A)five
B)six
C)ten
D)eleven
E)twelve
A)five
B)six
C)ten
D)eleven
E)twelve

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
68
Esters are formed from the reaction of an ether with a carboxylic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
69
What two compounds will react to give this amide? 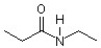
A)propanol and ammonia
B)propanoic acid and ammonia
C)propanoic acid and ethylamine
D)propanoic acid and diethylamine
E)propanoic acid and methylamine

A)propanol and ammonia
B)propanoic acid and ammonia
C)propanoic acid and ethylamine
D)propanoic acid and diethylamine
E)propanoic acid and methylamine

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
70
Methyl salicylate (oil of wintergreen)is used therapeutically as a counterirritant.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
71
Carboxylic acids with more than five carbons are very water soluble.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
72
Carboxylic acids are strong acids.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
73
Benzoic acid is an aliphatic carboxylic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
74
Acid catalyzed hydrolysis of an ester yields a carboxylic acid and an alcohol.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
75
An ester is derived from an alcohol and a carboxylic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
76
The major acidic component of vinegar is formic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
77
Propanoic acid is more soluble than pentanoic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
78
Carboxylic acids are responsible for the sweet taste of fruits and vegetables.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
79
Aspirin that has a smell of vinegar has broken down by hydrolysis.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
80
Carboxylic acids with four or fewer carbons are very water soluble.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck


