Deck 8: Reaction Rates and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 8: Reaction Rates and Equilibrium
1
Which is an incorrect statement when discussing molecular collisions leading to the chemical reaction,AB + CD AD + CB?
A)The faster the motion of the molecules,the more likely they are to collide.
B)The faster the motion of the molecules,the greater the probability that a chemical reaction will occur.
C)The slower the motion of the molecules,the more likely that this reaction will become an equilibrium reaction favoring the left side of the equation.
D)The relationship between molecular collisions and reaction rate is direct.
A)The faster the motion of the molecules,the more likely they are to collide.
B)The faster the motion of the molecules,the greater the probability that a chemical reaction will occur.
C)The slower the motion of the molecules,the more likely that this reaction will become an equilibrium reaction favoring the left side of the equation.
D)The relationship between molecular collisions and reaction rate is direct.
The slower the motion of the molecules,the more likely that this reaction will become an equilibrium reaction favoring the left side of the equation.
2
At 20°C,a sample of solid spontaneously sublimes to a gas.This change in state is accompanied by which of the following changes in the solid sample?
A)entropy & energy decrease
B)entropy & energy increase
C)entropy decreases & energy increases
D)energy decreases & entropy increases
A)entropy & energy decrease
B)entropy & energy increase
C)entropy decreases & energy increases
D)energy decreases & entropy increases
entropy & energy increase
3
Which of the following interpretations of the spontaneous chemical reaction is correct? V + W Y + Z + heat
A)V is written first because it contains more energy than W,Y,or Z.
B)The entropy on the left of the equation is greater than that on the right.
C)The entropy on the right of the equation is greater than that on the left.
D)Entropy has nothing to do with this chemical equation.
A)V is written first because it contains more energy than W,Y,or Z.
B)The entropy on the left of the equation is greater than that on the right.
C)The entropy on the right of the equation is greater than that on the left.
D)Entropy has nothing to do with this chemical equation.
The entropy on the right of the equation is greater than that on the left.
4
Which of the conditions given is necessary for a chemical reaction to occur?
A)The molecules of the reacting chemicals must be in motion.
B)The molecules of the reacting chemicals must bump into each other.
C)The molecules of the reacting chemicals must be of opposite charges.
D)The molecules of the reacting chemicals must be at different charges.
A)The molecules of the reacting chemicals must be in motion.
B)The molecules of the reacting chemicals must bump into each other.
C)The molecules of the reacting chemicals must be of opposite charges.
D)The molecules of the reacting chemicals must be at different charges.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
The energy required to start some spontaneous processes is called ___ .
A)internal energy
B)collision energy
C)free energy
D)activation energy
A)internal energy
B)collision energy
C)free energy
D)activation energy

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
Which is assumed to be true in most explanations of how reactions occur?
A)reactant particles must collide with each other
B)catalysts must be present
C)energy must be absorbed as the reaction proceeds
D)more than one response is correct
A)reactant particles must collide with each other
B)catalysts must be present
C)energy must be absorbed as the reaction proceeds
D)more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
In an exergonic process,the system _____ .
A)gains energy
B)loses energy
C)either gains or loses energy
D)no energy change at all
A)gains energy
B)loses energy
C)either gains or loses energy
D)no energy change at all

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Homogeneous catalysts are thought to function by
A)raising the temperature.
B)lowering activation energy of a reaction.
C)removing a reacting molecule.
D)More than one response is correct.
A)raising the temperature.
B)lowering activation energy of a reaction.
C)removing a reacting molecule.
D)More than one response is correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Catalytic action is
A)responsible for speeding up and slowing down chemical reactions.
B)directly opposed by inhibitors.
C)much more effective at room temperature than higher temperature.
D)there is more than one correct response.
A)responsible for speeding up and slowing down chemical reactions.
B)directly opposed by inhibitors.
C)much more effective at room temperature than higher temperature.
D)there is more than one correct response.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction conditions for a specific reaction,BCD + HA ABCDH,are correct,but the reaction does not occur.What could be the reason?
A)There are no collisions between the molecules.
B)The collisions of the particles with the container walls remove the required energy because it is transferred to the container molecules.
C)The orientation of the molecules with respect to each other is not correct for the reaction to occur.
D)There is more than one answer.
A)There are no collisions between the molecules.
B)The collisions of the particles with the container walls remove the required energy because it is transferred to the container molecules.
C)The orientation of the molecules with respect to each other is not correct for the reaction to occur.
D)There is more than one answer.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
For the reaction below,which of the following would take place once pure A and B are mixed together? A + B  C
C
A)The concentration of C would increase for a time,then remain constant.
B)The concentration of A would increase for a time,then decrease.
C)The concentration of B would increase for a time,then remain constant.
D)More than one response is correct.
 C
CA)The concentration of C would increase for a time,then remain constant.
B)The concentration of A would increase for a time,then decrease.
C)The concentration of B would increase for a time,then remain constant.
D)More than one response is correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an endergonic process?
A)exothermic reactions
B)endothermic reactions
C)equilibrium reactions
D)none of them
A)exothermic reactions
B)endothermic reactions
C)equilibrium reactions
D)none of them

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following states of matter generally has the lowest entropy?
A)a crystalline solid
B)a liquid
C)a gas
D)two have the same entropy
A)a crystalline solid
B)a liquid
C)a gas
D)two have the same entropy

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
Most automobiles function by burning gasoline by a process similar to the equation shown,in which octane is burned. C8H18 + O2 CO2 + H2O + heat (not balanced)
Using the concept of entropy,interpret the implications of this reaction.
A)It goes to the right because it is spontaneous after a spark is supplied.
B)It explains why a car stops running when there is no gas in the fuel tank.
C)Gasoline can be made commercially because the reaction can run backwards if the energy is returned to the CO2 and H2O.
D)All of these responses are correct with respect to entropy.
Using the concept of entropy,interpret the implications of this reaction.
A)It goes to the right because it is spontaneous after a spark is supplied.
B)It explains why a car stops running when there is no gas in the fuel tank.
C)Gasoline can be made commercially because the reaction can run backwards if the energy is returned to the CO2 and H2O.
D)All of these responses are correct with respect to entropy.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following will influence all reaction rates?
A)the presence of catalysts
B)the temperature of reactants
C)the concentration of reactants
D)more than one response is correct
A)the presence of catalysts
B)the temperature of reactants
C)the concentration of reactants
D)more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
A reaction is spontaneous when the conditions are
A)present for that reaction to progress and it does.
B)at STP.
C)such for an exothermic reaction that there is less energy in the reactants than in the products.
D)There is more than one correct response.
A)present for that reaction to progress and it does.
B)at STP.
C)such for an exothermic reaction that there is less energy in the reactants than in the products.
D)There is more than one correct response.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
Sulfur reacts with oxygen to produce sulfur dioxide,but only if the high activation energy is supplied.Which condition will lower the activation energy the most?
A)stirring the mixture
B)heating the mixture
C)using a catalyst
D)activation energy cannot be lowered
A)stirring the mixture
B)heating the mixture
C)using a catalyst
D)activation energy cannot be lowered

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
The concept of entropy
A)is employed to explain how energy can be stored.
B)is employed to explain how an exothermic reaction can become endothermic.
C)is employed as an indicator of disorder in a system.
D)explains why most chemical reactions are endothermic.
A)is employed to explain how energy can be stored.
B)is employed to explain how an exothermic reaction can become endothermic.
C)is employed as an indicator of disorder in a system.
D)explains why most chemical reactions are endothermic.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is most closely related to the term "reaction rate"?
A)the temperature needed to initiate a reaction
B)the position of equilibrium when a reaction stops
C)the speed of a reaction
D)more than one response is correct
A)the temperature needed to initiate a reaction
B)the position of equilibrium when a reaction stops
C)the speed of a reaction
D)more than one response is correct

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
What is the direct cause of a chemical reaction of the type E + F EF?
A)Catalysts are the direct cause of chemical reactions of this type.
B)The energy in the environment at room temperature causes the reaction.
C)The electromagnetic attraction between E and F draw them together.
D)Collisions occurring between E's and F's lead them to reaction.
A)Catalysts are the direct cause of chemical reactions of this type.
B)The energy in the environment at room temperature causes the reaction.
C)The electromagnetic attraction between E and F draw them together.
D)Collisions occurring between E's and F's lead them to reaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
Which statement is incorrect with reference to Le Châtelier's principle?
A)In a reaction of the type,Q + 2R 3T,the addition of one mole of Q results in the tripling of T.
3T,the addition of one mole of Q results in the tripling of T.
B)If the reaction to the right is exothermic,the reaction to the left must be endothermic.
C)If the reaction rate of the forward reaction is known,then the reaction rate of the reverse reaction is also known.
D)Responses b and c are incorrect.
A)In a reaction of the type,Q + 2R
 3T,the addition of one mole of Q results in the tripling of T.
3T,the addition of one mole of Q results in the tripling of T.B)If the reaction to the right is exothermic,the reaction to the left must be endothermic.
C)If the reaction rate of the forward reaction is known,then the reaction rate of the reverse reaction is also known.
D)Responses b and c are incorrect.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
In a system of equilibrium,it is true that the rate of the forward reaction
A)exceeds that of the reverse.
B)is lower than that of the reverse reaction.
C)is equal to the rate of the reverse reaction.
D)More than one response is correct.
A)exceeds that of the reverse.
B)is lower than that of the reverse reaction.
C)is equal to the rate of the reverse reaction.
D)More than one response is correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
An inhibitor will have what effect on a reaction?
A)increase the activation energy
B)lower the activation energy
C)slow the reaction down
D)lower the amount of product produced
A)increase the activation energy
B)lower the activation energy
C)slow the reaction down
D)lower the amount of product produced

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement is a correct application of Le Châtelier's principle?
A)Addition of a substance to the left side of an equilibrium shifts it to the right.
B)Any stress to the right side of a chemical equilibrium results in a shift to the left to relieve the stress.
C)Heat added or taken away from a chemical equilibrium in which one side is exothermic will not shift the reaction.
D)Responses b and c are correct.
A)Addition of a substance to the left side of an equilibrium shifts it to the right.
B)Any stress to the right side of a chemical equilibrium results in a shift to the left to relieve the stress.
C)Heat added or taken away from a chemical equilibrium in which one side is exothermic will not shift the reaction.
D)Responses b and c are correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
Which statement applies to a reaction at equilibrium?
A)The energy of activation has been supplied to bring the reaction to equilibrium.
B)The state of the equilibrium can be expressed as a ratio.
C)There are equal amounts of matter on either side of the reaction.
D)All of the responses are correct.
A)The energy of activation has been supplied to bring the reaction to equilibrium.
B)The state of the equilibrium can be expressed as a ratio.
C)There are equal amounts of matter on either side of the reaction.
D)All of the responses are correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
When the equilibrium expression is written for the reaction N2(g)+ 3F2(g)  2NF3(g)
2NF3(g)
What is the exponent of the concentration of fluorine,F2?
A)1
B)2
C)3
D)0
 2NF3(g)
2NF3(g)What is the exponent of the concentration of fluorine,F2?
A)1
B)2
C)3
D)0

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
Equilibrium:
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O CH3OH + H2 + heat
CH3OH + H2 + heat
Indicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Add a catalyst to the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O
 CH3OH + H2 + heat
CH3OH + H2 + heatIndicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Add a catalyst to the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
A catalyzed,aqueous chemical reaction occurs.Which of the following conditions will reach completion the soonest?
A)a low concentration of catalyst
B)a high concentration of the reactants
C)a high concentration of catalyst
D)stirring the reaction mixture
A)a low concentration of catalyst
B)a high concentration of the reactants
C)a high concentration of catalyst
D)stirring the reaction mixture

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
CFC's are in the environment because they were released into the atmosphere.What was the use of CFC's?
A)These are Chlorine-Fluorine Chain compounds used to purify the water.
B)CFC's were used in pressurized cans that dispersed hair spray,paint,and other sprayed materials.
C)CFC's had a use in the manufacture of steel and were released to the atmosphere by large industry.
D)There is more than one correct response.
A)These are Chlorine-Fluorine Chain compounds used to purify the water.
B)CFC's were used in pressurized cans that dispersed hair spray,paint,and other sprayed materials.
C)CFC's had a use in the manufacture of steel and were released to the atmosphere by large industry.
D)There is more than one correct response.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
Equilibrium:
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O CH3OH + H2 + heat
CH3OH + H2 + heat
Indicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Cool the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O
 CH3OH + H2 + heat
CH3OH + H2 + heatIndicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Cool the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
The equilibrium for the following reaction at 1 atm and 25°C lies far to the left.A possible equilbrium constant for this reaction would be _____ . N2 + 3H2  3NH3
3NH3
A)0.5
B)0
C)1.5
D)impossible to predict K.
 3NH3
3NH3A)0.5
B)0
C)1.5
D)impossible to predict K.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
Equilibrium:
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O CH3OH + H2 + heat
CH3OH + H2 + heat
Indicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Add CH4 to the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O
 CH3OH + H2 + heat
CH3OH + H2 + heatIndicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Add CH4 to the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements about a K of 3.5 calculated for a specific chemical reaction at equilibrium is incorrect?
A)It was calculated by dividing the product of the products by that of the reactants.
B)It indicates that one side of the reaction has most of the matter present.
C)It is an evaluation of the extent of the two chemical reactions involved: the forward and the reverse reactions.
D)A K of 3.5 indicates that a catalyst will push the reaction to the right.
A)It was calculated by dividing the product of the products by that of the reactants.
B)It indicates that one side of the reaction has most of the matter present.
C)It is an evaluation of the extent of the two chemical reactions involved: the forward and the reverse reactions.
D)A K of 3.5 indicates that a catalyst will push the reaction to the right.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
Equilibrium:
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O CH3OH + H2 + heat
CH3OH + H2 + heat
Indicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Remove H2 from the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left
The following question(s)refer to the equilibrium given below in which all reactants and products are gases.
CH4 + H2O
 CH3OH + H2 + heat
CH3OH + H2 + heatIndicate the effect of the changing condition on the position of equilibrium.
Refer to Equilibrium.Remove H2 from the mixture.
A)shifts left
B)shifts right
C)no effect
D)can shift to right or left

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
BrCl is put into an empty 1.00 L container.At equilibrium,the molar concentration of BrCl is 0.382,and that of Br2 is 0.319.Evaluate the equilibrium constant for the following reaction. Br2(g)+ Cl2(g)  2BrCl(g)
2BrCl(g)
A)0.457
B)0.699
C)1.43
D)5.74
 2BrCl(g)
2BrCl(g)A)0.457
B)0.699
C)1.43
D)5.74

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Which statement applies to equilibria in general?
A)The larger the value of K,the closer the amounts of matter on the right and left will be equal.
B)The larger the value of K,the greater the amount of matter on the right.
C)The larger the value of K,the greater the amount of matter on the left.
D)None of these responses are correct because reactions at equilibrium have equal amounts of matter on the left and the right sides of the equation.
A)The larger the value of K,the closer the amounts of matter on the right and left will be equal.
B)The larger the value of K,the greater the amount of matter on the right.
C)The larger the value of K,the greater the amount of matter on the left.
D)None of these responses are correct because reactions at equilibrium have equal amounts of matter on the left and the right sides of the equation.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the statements is correct when referring to the following reaction? H2(g)+ I2(g)  2HI(g)
2HI(g)
A)This reaction is reversible because the amount of heat given off by the forward reaction is the same as the heat given off by the reverse reaction.
B)The physical state of the reaction is found by using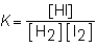 .
.
C)Most of the matter is on the right side of the equation.
D)There isn't a correct response provided.
 2HI(g)
2HI(g)A)This reaction is reversible because the amount of heat given off by the forward reaction is the same as the heat given off by the reverse reaction.
B)The physical state of the reaction is found by using
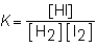 .
.C)Most of the matter is on the right side of the equation.
D)There isn't a correct response provided.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
CFC's such as CF2Cl2,are associated with the increase in chemical activity that degrades ozone.Which of the following relates to this phenomenon?
A)This is not serious because the only area of the planet involved is at the poles and there is very little life to be affected by the increase in UV.
B)The catalyzing of the ozone breakdown decreases the amount of UV at the surface of the planet.
C)The process by which the ozone is degraded is catalytic and can be controlled by the reduction of CFC's.
D)All of these responses are correct.
A)This is not serious because the only area of the planet involved is at the poles and there is very little life to be affected by the increase in UV.
B)The catalyzing of the ozone breakdown decreases the amount of UV at the surface of the planet.
C)The process by which the ozone is degraded is catalytic and can be controlled by the reduction of CFC's.
D)All of these responses are correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Which method can be used to product a timed-release medication?
A)The patient is given the medication in small doses over time,rather than one larger dose.
B)The patient is to take the medication two or three times a day.
C)The medication is provided in special capsules that are subdivided into smaller,pulverized portions of the medicinal compound.
D)The medication compound is produced in very small amounts and each is coated with a material of varying thickness.
A)The patient is given the medication in small doses over time,rather than one larger dose.
B)The patient is to take the medication two or three times a day.
C)The medication is provided in special capsules that are subdivided into smaller,pulverized portions of the medicinal compound.
D)The medication compound is produced in very small amounts and each is coated with a material of varying thickness.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following products is not suitable to timed-release method for medications that are desired to have an extended effective level time?
A)appetite control materials
B)analgesics used for middle-aged people
C)fluoride compounds in a toothpaste
D)diuretics
A)appetite control materials
B)analgesics used for middle-aged people
C)fluoride compounds in a toothpaste
D)diuretics

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
For the following reaction,which of the given changes will increase the value of the equilibrium constant? AB + CD  AD + BC + heat
AD + BC + heat
A)decreasing the temperature
B)adding AB to the reaction
C)removing CD from the reaction
D)All of these changes will increase K.
 AD + BC + heat
AD + BC + heatA)decreasing the temperature
B)adding AB to the reaction
C)removing CD from the reaction
D)All of these changes will increase K.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
If we add a catalyst to the following equation,which way will the equilibrium shift? CO + H2O + heat  CO2 + H2
CO2 + H2
A)to the left
B)will have no effect
C)to the right
D)not enough information.
 CO2 + H2
CO2 + H2A)to the left
B)will have no effect
C)to the right
D)not enough information.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
Ice has a(n)____ entropy than that of liquid water.
A)lower
B)equal
C)higher
D)can not tell
A)lower
B)equal
C)higher
D)can not tell

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
For the following energy diagram,which letter represents the catalyzed activation energy? 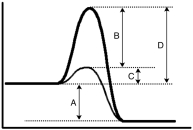
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
You've been told that 'when a change is made in any factor of an established equilibrium,the position of equilibrium will shift in a direction that will minimize or oppose the change.' This is a statement of which of the following?
A)molecular collision principle
B)Le Châtelier's principle
C)law of definite proportions
D)Boyle's law
A)molecular collision principle
B)Le Châtelier's principle
C)law of definite proportions
D)Boyle's law

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
A patient complains about having painful muscle spasms after working on a very hot day.The patient's skin is moist and cool,heart rate is near normal as well as his/her body temperature.What should you not do for them?
A)get them to a cool place
B)give them a caffeinated drink
C)sponge them with cool water
D)encourage them to lie down and rest
A)get them to a cool place
B)give them a caffeinated drink
C)sponge them with cool water
D)encourage them to lie down and rest

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
A process that gains or accepts energy as it takes place is a(n)_____ .
A)exergonic process
B)spontaneous process
C)endergonic process
D)all of these
A)exergonic process
B)spontaneous process
C)endergonic process
D)all of these

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
What would be the effect of doubling the pressure on the following equilibrium system? N2(g)+ 3F2(g)  2NF3(g)
2NF3(g)
A)Shift it to the right.
B)Shift it to the left.
C)Will have no effect.
D)Will be impossible to predict.
 2NF3(g)
2NF3(g)A)Shift it to the right.
B)Shift it to the left.
C)Will have no effect.
D)Will be impossible to predict.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is the proper equilibrium expression for the reaction shown below? 2A + B  C + heat
C + heat
A)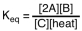
B)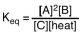
C)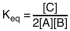
D)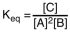
 C + heat
C + heatA)
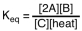
B)
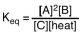
C)
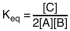
D)
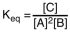

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
A doctor has given your patient a prescription of a common amphetamine.What should the patient avoid when taking this medication?
A)exercise
B)fruit juices
C)alcohol
D)none of the choices
A)exercise
B)fruit juices
C)alcohol
D)none of the choices

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
The equilibrium constants for a series of reaction of the general type: AB + CD  AD + BC
AD + BC
Were measured and are shown below.In which reaction is the concentration of the products the largest at equilibrium?
A)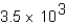
B)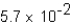
C)0.011
D)1.2
 AD + BC
AD + BCWere measured and are shown below.In which reaction is the concentration of the products the largest at equilibrium?
A)
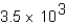
B)
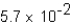
C)0.011
D)1.2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Based upon the energy diagram given below,which letter represents the enthalpy for the reaction? 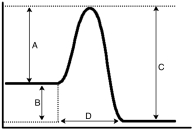
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
A room filled with a 2:1 mixture of hydrogen and oxygen gases can be safely inhaled.However,with a single spark,it can react explosively.Since the explosion would be considered a spontaneous process,what did the spark provide?
A)endothermic energy
B)exothermic energy
C)free energy
D)activation energy
A)endothermic energy
B)exothermic energy
C)free energy
D)activation energy

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is NOT true regarding a chemical reaction?
A)All matter is considered to be composed of tiny particles.
B)All particles contain potential energy,measured by constant motion.
C)Particle speed always increases with rising temperature.
D)Colliding particles will transfer energy with no loss in system energy.
A)All matter is considered to be composed of tiny particles.
B)All particles contain potential energy,measured by constant motion.
C)Particle speed always increases with rising temperature.
D)Colliding particles will transfer energy with no loss in system energy.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
The device used to measure heat produced or used by a chemical reaction is called a(n)_____ .
A)thermometer
B)calorimeter
C)activator
D)joulery
A)thermometer
B)calorimeter
C)activator
D)joulery

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the following energy diagram.Which letter represents the activation energy for the forward reaction? 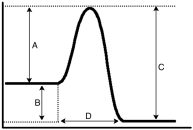
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
An elderly person comes to you on a winter day with blue fingers and toes,sluggish movement,and slurred speech.What condition do they most likely have?
A)hyperthermia
B)hypothermia
C)heat exhaustion
D)none of the choices
A)hyperthermia
B)hypothermia
C)heat exhaustion
D)none of the choices

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
If we remove CO2 from the following equation,which way will the equilibrium shift? CO + H2O + heat  CO2 + H2
CO2 + H2
A)to the left
B)will have no effect
C)to the right
D)not enough information
 CO2 + H2
CO2 + H2A)to the left
B)will have no effect
C)to the right
D)not enough information

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
The following reaction is observed in a lab experiment: A + 2B C + D In this experiment,it required 750 s for the concentration of C to change from 0.333 M to 0.750 M.What is the rate of the reaction?
A)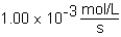
B)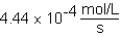
C)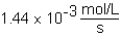
D)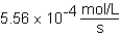
A)
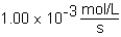
B)
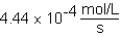
C)
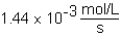
D)
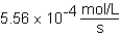

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following would be the equilibrium expression for the system given below? N2(g)+ 3F2(g)  2NF3(g)
2NF3(g)
A)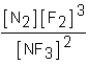
B)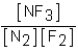
C)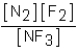
D)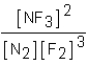
 2NF3(g)
2NF3(g)A)
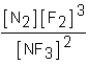
B)
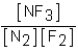
C)
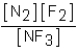
D)
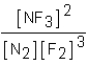

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
Increasing the concentration of a reactant will increase the number of effective collisions.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
A reaction rate can be described in terms of the change in concentration of either a reactant or a product.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
Catalysts may lower the activation energy.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
Reactions that have a low energy of activation tend to proceed at a high rate.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction below does not progress at room temperature,but does at 1100 C.Therefore,this reaction is spontaneous at 1100 C. 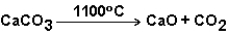


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
A reaction will occur each time two reactant molecules collide.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
A spontaneous process accompanied by an entropy decrease must also be accompanied by an energy decrease.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
Chemical reactions always occur once the molecules of two substances collide.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
The effect of a catalyst is to eliminate the activation energy requirement of chemical reactions.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
Butane,C4H10,burning in air,is an example of a substance combining with oxygen to produce CO2 and H2O.If pure oxygen were to be supplied,the rate of combustion would increase significantly.Then there is more energy given off when 10 g of butane burns in pure oxygen,rather than in air.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
A reaction rate can be described using any unit of time (seconds,hours,etc.).

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
When a spontaneous process in accompanied by an energy increase,then a large entropy increase must also occur.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
You purchase a glow stick during an evening parade.After it ends,your roommate tells you that if you place it in the freezer,it will glow for a longer time.Assuming she knows what she is talking about,why would this be true?
A)Reaction rates can be expected to decrease as temperature is reduced.
B)It's dark in a closed freezer,preventing the stick from glowing.
C)There is less oxygen inside the freezer than outside,so the reaction slows down.
D)Your roommate simply made up the story to annoy you.
A)Reaction rates can be expected to decrease as temperature is reduced.
B)It's dark in a closed freezer,preventing the stick from glowing.
C)There is less oxygen inside the freezer than outside,so the reaction slows down.
D)Your roommate simply made up the story to annoy you.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
Reaction rates are determined experimentally.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
The products of an exothermic reaction contain less energy than do the reactants.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
If heat is absorbed when a compound dissolves in water,the compound should be more soluble at higher temperatures.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Decreasing the temperature will decrease the number of effective collisions.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
Activation energy is the total energy released when a reaction takes place.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
A heterogeneous catalyst will normally dissolve in the reaction mixture.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
Enthalpy always increases with increasing randomness.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


