Deck 4: Forces Between Particles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 4: Forces Between Particles
1
Some elements can display more than one charge.The most stable charge is
A)due to having 6 electrons in the outside orbit.
B)that charge produced by a loss of electrons,not a gain of electrons.
C)the charge produced by the noble gas configuration.
D)All are correct.
A)due to having 6 electrons in the outside orbit.
B)that charge produced by a loss of electrons,not a gain of electrons.
C)the charge produced by the noble gas configuration.
D)All are correct.
the charge produced by the noble gas configuration.
2
A covalent bond results when
A)one atom gives up an electron to another atom.
B)one atom gives up two electrons to another atom.
C)two atoms share a pair of electrons.
D)two atoms share a single electron.
A)one atom gives up an electron to another atom.
B)one atom gives up two electrons to another atom.
C)two atoms share a pair of electrons.
D)two atoms share a single electron.
two atoms share a pair of electrons.
3
For some representative metals,two possible ions can be formed.Why?
A)s electrons can fill the next lower d orbital.
B)They can lose electrons to form a cation and gain electrons to form an anion.
C)They can lose the s electrons or all electrons in their outer shell.
D)They can lose just the p electrons or all electrons in their outer shell.
A)s electrons can fill the next lower d orbital.
B)They can lose electrons to form a cation and gain electrons to form an anion.
C)They can lose the s electrons or all electrons in their outer shell.
D)They can lose just the p electrons or all electrons in their outer shell.
They can lose just the p electrons or all electrons in their outer shell.
4
The ionic compound that forms between magnesium (Mg)and oxygen (O)has the formula _____ .
A)Mg2O
B)MgO
C)MgO2
D)Mg2O3
A)Mg2O
B)MgO
C)MgO2
D)Mg2O3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following would you expect to be the most polar molecule?
A)CO2
B)H2S
C)KCl
D)SiH4
A)CO2
B)H2S
C)KCl
D)SiH4

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
A correct Lewis structure for BrCl is _____ .
A)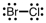
B)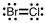
C)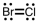
D)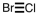
A)
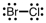
B)
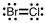
C)
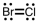
D)
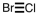

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
The rigid three-dimensional arrangement assumed by the ions of ionic compounds is called a
A)molecule.
B)binary compound.
C)lattice site.
D)crystal lattice.
A)molecule.
B)binary compound.
C)lattice site.
D)crystal lattice.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following distinguishing electron configurations is characteristic of noble gases?
A)nf14
B)nd10
C)np6
D)ns2
A)nf14
B)nd10
C)np6
D)ns2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following electronic changes will cause a Group VA (15)element to achieve a noble gas configuration?
A)gain three electrons
B)gain two electrons
C)gain one electron
D)lose three electrons
A)gain three electrons
B)gain two electrons
C)gain one electron
D)lose three electrons

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
When magnesium forms an ion,it would be represented as _____ .
A)Mg+
B)Mg2+
C)Mg+.
D)Mg2+.
A)Mg+
B)Mg2+
C)Mg+.
D)Mg2+.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the correct Lewis structure for C2H4?
A)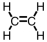
B)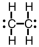
C)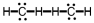
D)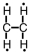
A)

B)

C)
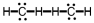
D)


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
If the electronegativity difference between A and B is 0.8,what type of bond is formed between the two elements?
A)polar covalent
B)nonpolar covalent
C)ionic
D)metallic
A)polar covalent
B)nonpolar covalent
C)ionic
D)metallic

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is correct with reference to covalent bonding?
A)Covalent bonds only form between like atoms.
B)The nonpolar covalent bond is the reason for hydrogen bonding.
C)Polyatomic compounds contain only covalent bonds.
D)None of these is a correct statement.
A)Covalent bonds only form between like atoms.
B)The nonpolar covalent bond is the reason for hydrogen bonding.
C)Polyatomic compounds contain only covalent bonds.
D)None of these is a correct statement.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following pairs of elements is most likely to form a covalent bond?
A)Fe H
B)Cs Br
C)Cl O
D)Zn S
A)Fe H
B)Cs Br
C)Cl O
D)Zn S

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
When an ionic compound forms between sodium (Na)and bromine (Br)atoms,it is true that
A)a bromine atom donates an electron to a sodium atom.
B)both the bromine atoms and sodium atoms donate electrons to each other.
C)a sodium atom donates an electron to a bromine atom.
D)neither bromine nor sodium atoms donate electrons to each other.
A)a bromine atom donates an electron to a sodium atom.
B)both the bromine atoms and sodium atoms donate electrons to each other.
C)a sodium atom donates an electron to a bromine atom.
D)neither bromine nor sodium atoms donate electrons to each other.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a correct Lewis structure for a group IIIA(13)element M?
A)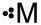
B)
C)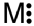
D)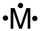
A)
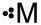
B)

C)
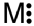
D)
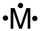

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the highest electronegativity?
A)O
B)N
C)Si
D)P
A)O
B)N
C)Si
D)P

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following would cause a calcium atom (Ca)to achieve a noble gas configuration?
A)lose one electron
B)lose two electrons
C)gain one electron
D)gain two electrons
A)lose one electron
B)lose two electrons
C)gain one electron
D)gain two electrons

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
The correct name for CeS is _____ .
A)cesium sulfate
B)cesium sulfide
C)cerium sulfate
D)cerium sulfide
A)cesium sulfate
B)cesium sulfide
C)cerium sulfate
D)cerium sulfide

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following has a noble gas electronic configuration?
A)Cl
B)O-
C)Br-
D)Cl+
A)Cl
B)O-
C)Br-
D)Cl+

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
A polar molecule must have
A)polar bonds.
B)an unsymmetrical arrangement.
C)neither polar bonds nor an unsymmetrical arrangement.
D)both polar bonds and an unsymmetrical arrangement.
A)polar bonds.
B)an unsymmetrical arrangement.
C)neither polar bonds nor an unsymmetrical arrangement.
D)both polar bonds and an unsymmetrical arrangement.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
The correct name for KHPO4 is _____ .
A)potassium phosphate
B)monopotassium phosphate
C)potassium hydrogen phosphate
D)KHPO4 is not a compound
A)potassium phosphate
B)monopotassium phosphate
C)potassium hydrogen phosphate
D)KHPO4 is not a compound

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
The correct formula for the ionic compound containing Al3+ and PO 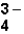 would be _____.
would be _____.
A)Al3(PO4)3
B)AlPO4
C)Al3(PO4)2
D)Al2(PO4)3
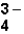 would be _____.
would be _____.A)Al3(PO4)3
B)AlPO4
C)Al3(PO4)2
D)Al2(PO4)3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
Which interparticle forces would you expect to find in a sample of the element lithium?
A)covalent
B)dispersion
C)ionic
D)metallic
A)covalent
B)dispersion
C)ionic
D)metallic

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
The molecular structure of water should be
A)linear because of the electron configuration of the oxygen atom.
B)flat triangular because water contains 3 atoms.
C)bent because of the unshared electrons on the molecule.
D)undetermined as there is no way to predict the shape of the water molecule.
A)linear because of the electron configuration of the oxygen atom.
B)flat triangular because water contains 3 atoms.
C)bent because of the unshared electrons on the molecule.
D)undetermined as there is no way to predict the shape of the water molecule.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
The correct name for the covalent compound N2O4 is _____ .
A)dinitrogen tetroxide
B)nitrogen oxide
C)nitrogen quartoxide
D)dinitrogen quartoxide
A)dinitrogen tetroxide
B)nitrogen oxide
C)nitrogen quartoxide
D)dinitrogen quartoxide

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
The correct name for K2SO4 is _____ .
A)potassium sulfate
B)potassium sulfide
C)potassium sulfite
D)potassium tetrasulfoxygen
A)potassium sulfate
B)potassium sulfide
C)potassium sulfite
D)potassium tetrasulfoxygen

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
Which type of interparticle forces leads to compounds with the lowest melting points?
A)covalent
B)dispersion
C)ionic
D)dipole-dipole
A)covalent
B)dispersion
C)ionic
D)dipole-dipole

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following correctly illustrates hydrogen bonding in water?
A)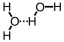
B)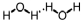
C)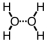
D)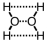
A)

B)
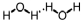
C)

D)


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the polyatomic ion call carbonate?
A)C2Cl2 -
B)ClO -
C)CO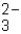
D)HCO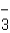
A)C2Cl2 -
B)ClO -
C)CO
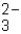
D)HCO

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
Hydrogen bonds form between the molecules of the hypothetical compound ABCH and do not form between DEFH molecules.
A)ABCH has a higher molecular weight than DEFH.
B)ABCH represents a compound that contains O,N,or F directly bonded to H.
C)ABCH tends to be a compound with color,but DEFH does not have color.
D)There is no significant difference between ABCH and DEFH.
A)ABCH has a higher molecular weight than DEFH.
B)ABCH represents a compound that contains O,N,or F directly bonded to H.
C)ABCH tends to be a compound with color,but DEFH does not have color.
D)There is no significant difference between ABCH and DEFH.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following would be a polar molecule?
A)O2
B)HCl
C)CH4
D)F2
A)O2
B)HCl
C)CH4
D)F2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
In describing the strength of interparticle forces,we discover that the weakest forces of bonds are _____ .
A)covalent
B)metallic
C)dipolar
D)dispersion
A)covalent
B)metallic
C)dipolar
D)dispersion

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
The correct formula of an ionic compound containing Fe2+ and ClO 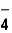 is _____ .
is _____ .
A)FeClO4
B)Fe2(ClO4)3
C)Fe(ClO4)2
D)Fe3(ClO4)2
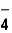 is _____ .
is _____ .A)FeClO4
B)Fe2(ClO4)3
C)Fe(ClO4)2
D)Fe3(ClO4)2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following covalent molecules contains polar bonds?
A)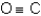
B)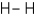
C)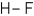
D)more than one response is correct
A)
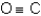
B)
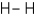
C)
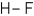
D)more than one response is correct

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecular geometries would most likely give rise to a nonpolar compound?
A)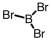
B)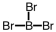
C)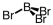
D)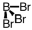
A)

B)
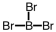
C)

D)
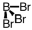

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
What single condition is required to insure that a molecule will be polar covalent?
A)a bond between like atoms
B)a bond between atoms that are different in electronegativity
C)a bond between atoms that are of widely different atomic weights
D)none of the choices
A)a bond between like atoms
B)a bond between atoms that are different in electronegativity
C)a bond between atoms that are of widely different atomic weights
D)none of the choices

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
The correct name for BaCO3 is _____ .
A)barium trioxide
B)barium monocarbon trioxide
C)barium carbonate
D)barium monocarbonate
A)barium trioxide
B)barium monocarbon trioxide
C)barium carbonate
D)barium monocarbonate

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
The shape of a BF3 molecule is _____ .
A)angular
B)trigonal planar
C)trigonal pyramid
D)tetrahedral
A)angular
B)trigonal planar
C)trigonal pyramid
D)tetrahedral

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
The correct formula for ammonium sulfate is _____ .
A)NH4SO4
B)(NH4)2SO4
C)NH4(SO4)2
D)(NH4)2(SO4)3
A)NH4SO4
B)(NH4)2SO4
C)NH4(SO4)2
D)(NH4)2(SO4)3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
The lattice site in a crystal is
A)the general location of all particles in a crystal.
B)the individual location occupied by a particle.
C)the rigid three-dimensional arrangement of particles.
D)None of the choices.
A)the general location of all particles in a crystal.
B)the individual location occupied by a particle.
C)the rigid three-dimensional arrangement of particles.
D)None of the choices.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
The most common positive ion in human fluids is _____ .
A)Cl-
B)PO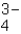
C)Na+
D)Fe2+
A)Cl-
B)PO
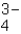
C)Na+
D)Fe2+

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
What is the formula mass of the compound formed between Al3+ and Cl-?
A)187.5 u
B)62.5 u
C)116.5 u
D)133.5 u
A)187.5 u
B)62.5 u
C)116.5 u
D)133.5 u

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
NH3 (ammonia)exhibits what type of structure?
A)triangle
B)pyramid with square base
C)pyramid with triangular base
D)none of these
A)triangle
B)pyramid with square base
C)pyramid with triangular base
D)none of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
The anion in an ionic compound whose name ends in "-ide" is
A)always a simple ion.
B)always a polyatomic ion.
C)either a simple or polyatomic ion.
D)a positive charge.
A)always a simple ion.
B)always a polyatomic ion.
C)either a simple or polyatomic ion.
D)a positive charge.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
Arrange the following bonds in order of increasing bond polarity.
A)C-O < P-O < Cl-O
B)Cl-O < C-O < P-O
C)P-O < Cl-O < C-O
D)C-O < Cl-O < P-O
A)C-O < P-O < Cl-O
B)Cl-O < C-O < P-O
C)P-O < Cl-O < C-O
D)C-O < Cl-O < P-O

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
In what part of the human body is nitric oxide,NO,produced?
A)the brain
B)the heart
C)blood vessels
D)the skin
A)the brain
B)the heart
C)blood vessels
D)the skin

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following correctly represents the polarity of the bond?
A)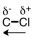
B)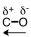
C)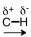
D)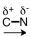
A)
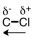
B)
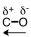
C)
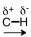
D)
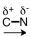

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
During the formation of an ionic bond between Na and N,which of the following reactions occurs?
A)
B)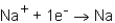
C)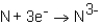
D)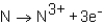
A)

B)
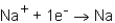
C)
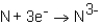
D)
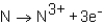

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
What would be the proper name for the following binary ionic compound CaBr2?
A)calcium dibromine
B)monocalcium bromine
C)monocalcium dibromide
D)calcium bromide
A)calcium dibromine
B)monocalcium bromine
C)monocalcium dibromide
D)calcium bromide

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
Which pair of elements will be further apart in electronegativity?
A)Cl and F
B)Li and Cl
C)Mn and Se
D)U and Cs
A)Cl and F
B)Li and Cl
C)Mn and Se
D)U and Cs

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following Lewis structures depicts provides a reasonable representation of SO2?
A)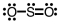
B)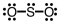
C)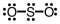
D)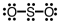
A)
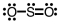
B)
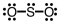
C)
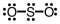
D)
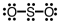

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
How many moles of K+ and P3- ions are present in 2.00 moles of K3P?
A)6.00 mol K+ and 2.00 mol P3-
B)3.00 mol K+ and 1.00 mol P3-
C)1.00 mol K+ and 3.00 mol P3-
D)2.00 mol K+ and 6.00 mol P3-
A)6.00 mol K+ and 2.00 mol P3-
B)3.00 mol K+ and 1.00 mol P3-
C)1.00 mol K+ and 3.00 mol P3-
D)2.00 mol K+ and 6.00 mol P3-

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following molecules would be classified as polar covalent?
A)NaCl
B)CH3Cl
C)KCl
D)CaCl2
A)NaCl
B)CH3Cl
C)KCl
D)CaCl2

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
Atoms or ions are considered isoelectronic if
A)they have similar electron configurations.
B)they have different number of electrons.
C)they have the same number of protons.
D)None of the choices.
A)they have similar electron configurations.
B)they have different number of electrons.
C)they have the same number of protons.
D)None of the choices.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
Which is the correct name for UF6?
A)uranyl fluoride
B)uranium hexafluoride
C)uranium (VI)fluoride
D)uranium (IV)fluoride
A)uranyl fluoride
B)uranium hexafluoride
C)uranium (VI)fluoride
D)uranium (IV)fluoride

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the following geometric shapes.Which one is a tetrahedral?
A)
B)
C)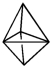
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following metals would not use a Roman Numeral as part of its name in a compound?
A)Cr
B)Mn
C)Co
D)Ca
A)Cr
B)Mn
C)Co
D)Ca

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following reactions would not produce an octet of electrons in the ion product?
A)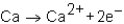
B)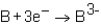
C)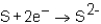
D)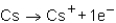
A)
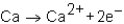
B)
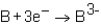
C)
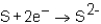
D)
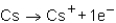

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
What medicinal value does ZnO have?
A)sun screen
B)relieves diaper rash
C)relieves poison ivy
D)all of these
A)sun screen
B)relieves diaper rash
C)relieves poison ivy
D)all of these

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
H2O is a binary compound.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
The water molecule has a bent structure.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
Metals gain electrons to become ions in ionic compounds.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
Both bonding and non-bonding electron pairs can influence molecular shape.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
Carbon dioxide is a linear molecule.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
Ammonia is a planar trigonal molecule.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
None of the noble gases make compounds.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
Only compounds containing hydrogen attached to an oxygen,nitrogen,or fluoride atom can exhibit hydrogen bonding.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
Knowing that indium is a member of group IIIA (13)and selenium is listed under group VIA (16),which of the following compounds should result from a reaction between these two elements?
A)InSe
B)In2Se3
C)In5Se3
D)Se5In3
A)InSe
B)In2Se3
C)In5Se3
D)Se5In3

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
HCl and HBr can exhibit hydrogen bonding with each other.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
K+ and Cl- have the same electronic configuration as argon.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
Fe2+ and Fe3+ are isoelectronic.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
Nitrogen is more electronegative than oxygen.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
Dispersion forces are among the strongest interparticle forces.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
Eating foods rich in _____ has been shown to help reduce hypertension.
A)chloride
B)iron
C)sodium
D)potassium
A)chloride
B)iron
C)sodium
D)potassium

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
The outer shell of a noble gas always contains eight electrons.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
Na+ and F- are isoelectronic.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
Oxygen must gain two electrons to achieve a noble gas configuration.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following would be considered as a rare earth?
A)cerium
B)cesium
C)curium
D)cadmium
A)cerium
B)cesium
C)curium
D)cadmium

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
CCl4 has a tetrahedral structure.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck


