Deck 12: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/113
Play
Full screen (f)
Deck 12: Chemical Kinetics
1
Consider the following rate law: 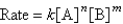 How are the exponents n and m determined?
How are the exponents n and m determined?
A)by using the balanced chemical equation
B)by using the subscripts for the chemical formulas
C)by using the coefficients of the chemical formulas
D)by educated guess
E)by experiment
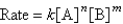 How are the exponents n and m determined?
How are the exponents n and m determined?A)by using the balanced chemical equation
B)by using the subscripts for the chemical formulas
C)by using the coefficients of the chemical formulas
D)by educated guess
E)by experiment
by experiment
2
Consider the reaction: 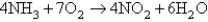 At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?
At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?
A)1.2 X
B)1.1 X
C)0.86 X
D)0.58 X
E)cannot be determined from the data
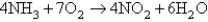 At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?
At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?A)1.2 X
B)1.1 X
C)0.86 X
D)0.58 X
E)cannot be determined from the data
0.86 X
3
The following questions refer to the reaction between nitric oxide and hydrogen
2NO + H2 N2O + H2O
![<strong>The following questions refer to the reaction between nitric oxide and hydrogen 2NO + H<sub>2</sub> \to N<sub>2</sub>O + H<sub>2</sub>O -What is the rate law for this reaction?</strong> A)Rate = k[NO] B)Rate = k[NO]<sup>2</sup> C)Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] D)Rate = k[NO][H<sub>2</sub>] E)Rate = k[N<sub>2</sub>O][H<sub>2</sub>O]](https://storage.examlex.com/TB6423/11efdc18_03f8_13df_8f65_a329fb6d5f14_TB6423_00.jpg)
-What is the rate law for this reaction?
A)Rate = k[NO]
B)Rate = k[NO]2
C)Rate = k[NO]2[H2]
D)Rate = k[NO][H2]
E)Rate = k[N2O][H2O]
2NO + H2 N2O + H2O
![<strong>The following questions refer to the reaction between nitric oxide and hydrogen 2NO + H<sub>2</sub> \to N<sub>2</sub>O + H<sub>2</sub>O -What is the rate law for this reaction?</strong> A)Rate = k[NO] B)Rate = k[NO]<sup>2</sup> C)Rate = k[NO]<sup>2</sup>[H<sub>2</sub>] D)Rate = k[NO][H<sub>2</sub>] E)Rate = k[N<sub>2</sub>O][H<sub>2</sub>O]](https://storage.examlex.com/TB6423/11efdc18_03f8_13df_8f65_a329fb6d5f14_TB6423_00.jpg)
-What is the rate law for this reaction?
A)Rate = k[NO]
B)Rate = k[NO]2
C)Rate = k[NO]2[H2]
D)Rate = k[NO][H2]
E)Rate = k[N2O][H2O]
Rate = k[NO]2[H2]
4
A general reaction written as A + 2B C + 2D is studied and yields the following data:

-What is the order of the reaction with respect to B?
A)0
B)1
C)2
D)3
E)4

-What is the order of the reaction with respect to B?
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
5
A general reaction written as A + 2B C + 2D is studied and yields the following data:

-What is the order of the reaction with respect to A?
A)0
B)1
C)2
D)3
E)4

-What is the order of the reaction with respect to A?
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the initial rate of B consumption ( [B]/ t)for the first trial?
A)8.00 10-3 mol/L·s
B)1.60 10-2 mol/L·s
C)3.20 10-2 mol/L·s
D)4.00 10-3 mol/L·s
E)none of these (A-D)
A)8.00 10-3 mol/L·s
B)1.60 10-2 mol/L·s
C)3.20 10-2 mol/L·s
D)4.00 10-3 mol/L·s
E)none of these (A-D)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
7
What is the numerical value of the rate constant?
A)0.053
B)1.19
C)2.37
D)5.63
E)none of these (A-D)
A)0.053
B)1.19
C)2.37
D)5.63
E)none of these (A-D)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
8
Determine the initial rate of C production ( [C]/ t)if [A] = 0.200 M and [B] = 0.500 M.
A)4.74 10-2 mol/L·s
B)2.37 10-1 mol/L·s
C)1.19 10-1 mol/L·s
D)8.23 10-2 mol/L·s
E)none of these (A-D)
A)4.74 10-2 mol/L·s
B)2.37 10-1 mol/L·s
C)1.19 10-1 mol/L·s
D)8.23 10-2 mol/L·s
E)none of these (A-D)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
9
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 10CO2 + 8H2O

-What is the value of the rate constant?
A)2 105 M.s-1
B)2 105 M-2.s-1
C)200 M-1.s-1
D)200 M-2.s-1
E)2 10-4 M.s-1
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 10CO2 + 8H2O

-What is the value of the rate constant?
A)2 105 M.s-1
B)2 105 M-2.s-1
C)200 M-1.s-1
D)200 M-2.s-1
E)2 10-4 M.s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
10
Consider the following data concerning the equation:
H2O2 + 3I- + 2H+ I3- + 2H2O
![<strong>Consider the following data concerning the equation: H<sub>2</sub>O<sub>2</sub> + 3I<sup>-</sup> + 2H<sup>+</sup> \to I<sub>3</sub><sup>-</sup> + 2H<sub>2</sub>O -The rate law for this reaction is</strong> A)rate = k[H<sub>2</sub>O<sub>2</sub>][I<sup>-</sup>][H<sup>+</sup>] B)rate = k[H<sub>2</sub>O<sub>2</sub>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[H<sup>+</sup>]<sup>2</sup> C)rate = k[I<sup>-</sup>][H<sup>+</sup>] D)rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>] E)rate = k[H<sub>2</sub>O<sub>2</sub>][I<sup>-</sup>]](https://storage.examlex.com/TB6423/11efdc17_782a_bd8d_8f65_af94418a678e_TB6423_00.jpg)
-The rate law for this reaction is
A)rate = k[H2O2][I-][H+]
B)rate = k[H2O2]2[I-]2[H+]2
C)rate = k[I-][H+]
D)rate = k[H2O2][H+]
E)rate = k[H2O2][I-]
H2O2 + 3I- + 2H+ I3- + 2H2O
![<strong>Consider the following data concerning the equation: H<sub>2</sub>O<sub>2</sub> + 3I<sup>-</sup> + 2H<sup>+</sup> \to I<sub>3</sub><sup>-</sup> + 2H<sub>2</sub>O -The rate law for this reaction is</strong> A)rate = k[H<sub>2</sub>O<sub>2</sub>][I<sup>-</sup>][H<sup>+</sup>] B)rate = k[H<sub>2</sub>O<sub>2</sub>]<sup>2</sup>[I<sup>-</sup>]<sup>2</sup>[H<sup>+</sup>]<sup>2</sup> C)rate = k[I<sup>-</sup>][H<sup>+</sup>] D)rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>] E)rate = k[H<sub>2</sub>O<sub>2</sub>][I<sup>-</sup>]](https://storage.examlex.com/TB6423/11efdc17_782a_bd8d_8f65_af94418a678e_TB6423_00.jpg)
-The rate law for this reaction is
A)rate = k[H2O2][I-][H+]
B)rate = k[H2O2]2[I-]2[H+]2
C)rate = k[I-][H+]
D)rate = k[H2O2][H+]
E)rate = k[H2O2][I-]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
11
The average value for the rate constant k (without units)is
A)2710
B)2.74 104
C)137
D)108
E)none of these
A)2710
B)2.74 104
C)137
D)108
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
12
Consider the reaction 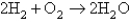 What is the ratio of the initial rate of the appearance of water to the initial rate of disappearance of oxygen?
What is the ratio of the initial rate of the appearance of water to the initial rate of disappearance of oxygen?
A)1 : 1
B)2 : 1
C)1 : 2
D)2 : 2
E)3 : 2
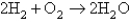 What is the ratio of the initial rate of the appearance of water to the initial rate of disappearance of oxygen?
What is the ratio of the initial rate of the appearance of water to the initial rate of disappearance of oxygen?A)1 : 1
B)2 : 1
C)1 : 2
D)2 : 2
E)3 : 2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
13
The following data were obtained for the reaction of NO with O2.Concentrations are in molecules/cm3 and rates are in molecules/cm3.s. ![<strong>The following data were obtained for the reaction of NO with O<sub>2</sub>.Concentrations are in molecules/cm<sup>3</sup> and rates are in molecules/cm<sup>3</sup>.s. What is the rate law?</strong> A)Rate = k[NO][O<sub>2</sub>] B)Rate = k[NO][O<sub>2</sub>]<sup>2</sup> C)Rate = k[NO]<sup>2</sup>[O<sub>2</sub>] D)Rate = k[NO]<sup>2</sup> E)Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]<sup>2</sup>](https://storage.examlex.com/TB6423/11efdc16_91f0_726b_8f65_6182a00a7fed_TB6423_00.jpg)
What is the rate law?
A)Rate = k[NO][O2]
B)Rate = k[NO][O2]2
C)Rate = k[NO]2[O2]
D)Rate = k[NO]2
E)Rate = k[NO]2[O2]2
![<strong>The following data were obtained for the reaction of NO with O<sub>2</sub>.Concentrations are in molecules/cm<sup>3</sup> and rates are in molecules/cm<sup>3</sup>.s. What is the rate law?</strong> A)Rate = k[NO][O<sub>2</sub>] B)Rate = k[NO][O<sub>2</sub>]<sup>2</sup> C)Rate = k[NO]<sup>2</sup>[O<sub>2</sub>] D)Rate = k[NO]<sup>2</sup> E)Rate = k[NO]<sup>2</sup>[O<sub>2</sub>]<sup>2</sup>](https://storage.examlex.com/TB6423/11efdc16_91f0_726b_8f65_6182a00a7fed_TB6423_00.jpg)
What is the rate law?
A)Rate = k[NO][O2]
B)Rate = k[NO][O2]2
C)Rate = k[NO]2[O2]
D)Rate = k[NO]2
E)Rate = k[NO]2[O2]2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
14
The balanced equation for the reaction of bromate ion with bromide ion in acidic solution is given by: 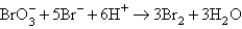 At a particular instant in time,the value of
At a particular instant in time,the value of 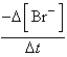 is
is 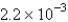 mol/L s.What is the value of
mol/L s.What is the value of 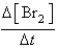 in the same units?
in the same units?
A)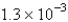
B)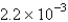
C)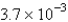
D)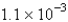
E)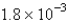
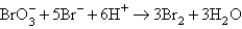 At a particular instant in time,the value of
At a particular instant in time,the value of 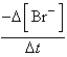 is
is 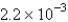 mol/L s.What is the value of
mol/L s.What is the value of 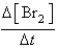 in the same units?
in the same units?A)
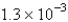
B)
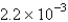
C)
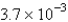
D)
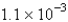
E)
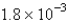

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the reaction X Y + Z Which of the following is a possible rate law?
A)Rate = k[X]
B)Rate = k[Y]
C)Rate = k[Y][Z]
D)Rate = k[X][Y]
E)Rate = k[Z]
A)Rate = k[X]
B)Rate = k[Y]
C)Rate = k[Y][Z]
D)Rate = k[X][Y]
E)Rate = k[Z]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
16
The average rate of disappearance of ozone in the reaction 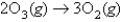 is found to be
is found to be 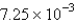 atm over a certain interval of time.What is the rate of appearance of
atm over a certain interval of time.What is the rate of appearance of 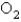 during this interval?
during this interval?
A)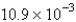 atm/s
atm/s
B)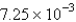 atm/s
atm/s
C)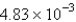 atm/s
atm/s
D)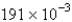 atm/s
atm/s
E)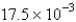 atm/s
atm/s
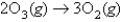 is found to be
is found to be 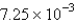 atm over a certain interval of time.What is the rate of appearance of
atm over a certain interval of time.What is the rate of appearance of 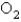 during this interval?
during this interval?A)
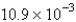 atm/s
atm/sB)
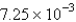 atm/s
atm/sC)
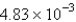 atm/s
atm/sD)
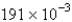 atm/s
atm/sE)
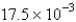 atm/s
atm/s
Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
17
Two mechanisms are proposed: I. 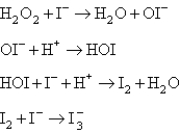 II.
II. 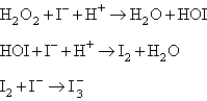 Which mechanism and which step as the rate determining step would best fit the data?
Which mechanism and which step as the rate determining step would best fit the data?
A)Mechanism I,with the first step the rate determining step.
B)Mechanism I,with the second step the rate determining step.
C)Mechanism II,with the first step rate determining.
D)Mechanism II,with the second step rate determining.
E)None of the above could be correct.
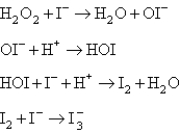 II.
II. 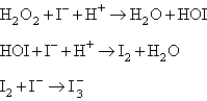 Which mechanism and which step as the rate determining step would best fit the data?
Which mechanism and which step as the rate determining step would best fit the data?A)Mechanism I,with the first step the rate determining step.
B)Mechanism I,with the second step the rate determining step.
C)Mechanism II,with the first step rate determining.
D)Mechanism II,with the second step rate determining.
E)None of the above could be correct.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
18
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 10CO2 + 8H2O
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> \to 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O -Which of the following is the correct rate law?</strong> A)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>5</sup>[H<sup>+</sup>]<sup>6</sup> B)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] C)Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] D)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>] E)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>2</sup>](https://storage.examlex.com/TB6423/11efdc17_af7d_2f7e_8f65_dfff33b37c4f_TB6423_00.jpg)
-Which of the following is the correct rate law?
A)Rate = k[MnO4-]2[H2C2O4]5[H+]6
B)Rate = k[MnO4-]2[H2C2O4][H+]
C)Rate = k[MnO4-][H2C2O4][H+]
D)Rate = k[MnO4-]2[H2C2O4]
E)Rate = k[MnO4-]2[H2C2O4]2
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 10CO2 + 8H2O
![<strong>The following initial rate data were found for the reaction 2MnO<sub>4</sub><sup>-</sup> + 5H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> + 6H<sup>+</sup> \to 2Mn<sup>2+</sup> + 10CO<sub>2</sub> + 8H<sub>2</sub>O -Which of the following is the correct rate law?</strong> A)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>5</sup>[H<sup>+</sup>]<sup>6</sup> B)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] C)Rate = k[MnO<sub>4</sub><sup>-</sup>][H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>][H<sup>+</sup>] D)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>] E)Rate = k[MnO<sub>4</sub><sup>-</sup>]<sup>2</sup>[H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>]<sup>2</sup>](https://storage.examlex.com/TB6423/11efdc17_af7d_2f7e_8f65_dfff33b37c4f_TB6423_00.jpg)
-Which of the following is the correct rate law?
A)Rate = k[MnO4-]2[H2C2O4]5[H+]6
B)Rate = k[MnO4-]2[H2C2O4][H+]
C)Rate = k[MnO4-][H2C2O4][H+]
D)Rate = k[MnO4-]2[H2C2O4]
E)Rate = k[MnO4-]2[H2C2O4]2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
19
For the reaction 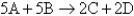 ,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?
,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?
A)-0.0223 M/s
B)0.112 M/s
C)-0.112 M/s
D)-0.00446 M/s
E)0.00446 M/s
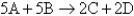 ,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?
,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?A)-0.0223 M/s
B)0.112 M/s
C)-0.112 M/s
D)-0.00446 M/s
E)0.00446 M/s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
20
A general reaction written as A + 2B C + 2D is studied and yields the following data:

-What is the overall order of the reaction?
A)0
B)1
C)2
D)3
E)4

-What is the overall order of the reaction?
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
21
The kinetics of the reaction ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of n?
A)0
B)0.5
C)1
D)1.5
E)2
![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of n?
A)0
B)0.5
C)1
D)1.5
E)2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
22
The kinetics of the reaction ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of m?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of m?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of m?
A)0
B)0.5
C)1
D)1.5
E)2
![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of m?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of m?</strong> A)0 B)0.5 C)1 D)1.5 E)2](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of m?
A)0
B)0.5
C)1
D)1.5
E)2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
23
What form will the pseudo-rate law have?
A)Rate = k'[A]x
B)Rate = k'[B]y
C)Rate = k'[A]x[B]y
D)Rate = kk'[A]x
E)Rate = kk'[B]y
A)Rate = k'[A]x
B)Rate = k'[B]y
C)Rate = k'[A]x[B]y
D)Rate = kk'[A]x
E)Rate = kk'[B]y

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
24
The following questions refer to the reaction between nitric oxide and hydrogen
2NO + H2 N2O + H2O

-What are the units for the rate constant for this reaction?
A)L/mol·s
B)L2/mol2·s
C)mol/L·s
D)s-2
E)L-2
2NO + H2 N2O + H2O

-What are the units for the rate constant for this reaction?
A)L/mol·s
B)L2/mol2·s
C)mol/L·s
D)s-2
E)L-2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction H2SeO3(aq)6I-(aq)+ 4H+(aq) 2I3-(aq)+ 3H2O(l)+ Se(s)was studied at 0°C by the method of initial rates:
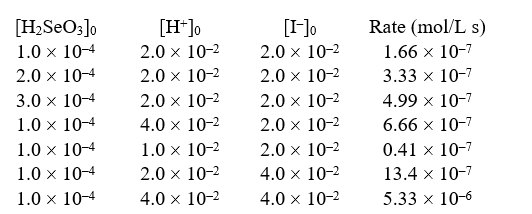
-The numerical value of the rate constant is
A)5.2 105
B)2.1 102
C)4.2
D)1.9 10-6
E)none of these
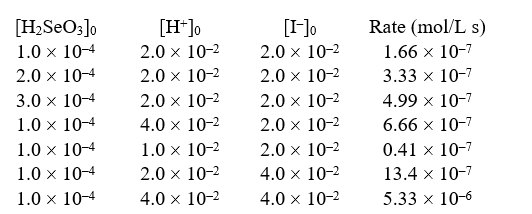
-The numerical value of the rate constant is
A)5.2 105
B)2.1 102
C)4.2
D)1.9 10-6
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
26
For the reaction 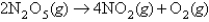 ,the following data were collected:
,the following data were collected:
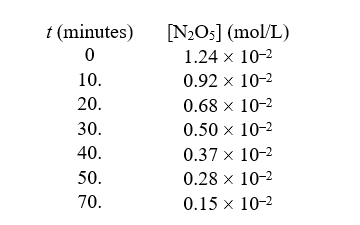
-The order of this reaction in N2O5 is
A)0
B)1
C)2
D)3
E)none of these
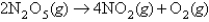 ,the following data were collected:
,the following data were collected:
-The order of this reaction in N2O5 is
A)0
B)1
C)2
D)3
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
27
The initial rate of production of NO2 for this reaction is approximately
A)7.4 10-4 mol/L·min
B)3.2 10-4 mol/L·min
C)1.24 10-2 mol/L·min
D)1.6 10-4 mol/L·min
E)none of these
A)7.4 10-4 mol/L·min
B)3.2 10-4 mol/L·min
C)1.24 10-2 mol/L·min
D)1.6 10-4 mol/L·min
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
28
The rate expression for a particular reaction is rate = k[A][B]2.If the initial concentration of B is increased from 0.1 M to 0.3 M,the initial rate will increase by which of the following factors?
A)2
B)6
C)12
D)3
E)9
A)2
B)6
C)12
D)3
E)9

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
29
For a reaction: ![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_a568_93a6_e75085a430a0_TB6423_11.jpg) ,[A]0 = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).
,[A]0 = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).
A)![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc79_93a6_bfb744e2e388_TB6423_11.jpg)
B)![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7a_93a6_57cd4d5f0f80_TB6423_11.jpg)
C)![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7b_93a6_ef27f06d29e1_TB6423_11.jpg)
D)![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7c_93a6_7faaee707a68_TB6423_11.jpg)
E)none of these
![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_a568_93a6_e75085a430a0_TB6423_11.jpg) ,[A]0 = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).
,[A]0 = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).A)
![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc79_93a6_bfb744e2e388_TB6423_11.jpg)
B)
![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7a_93a6_57cd4d5f0f80_TB6423_11.jpg)
C)
![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7b_93a6_ef27f06d29e1_TB6423_11.jpg)
D)
![<strong>For a reaction: ,[A]<sub>0</sub> = 4.2 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate k (without units).</strong> A) B) C) D) E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_cc7c_93a6_7faaee707a68_TB6423_11.jpg)
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
30
For which order reaction is the half-life of the reaction proportional to 1/k (k is the rate constant)?
A)zero order
B)first order
C)second order
D)all of the above
E)none of the above
A)zero order
B)first order
C)second order
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
31
The following questions refer to the reaction between nitric oxide and hydrogen
2NO + H2 N2O + H2O

-What is the order of this reaction?
A)3
B)2
C)1
D)0
E)cannot be determined from the data
2NO + H2 N2O + H2O

-What is the order of this reaction?
A)3
B)2
C)1
D)0
E)cannot be determined from the data

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
32
The half-life of this reaction is approximately
A)15 minutes
B)18 minutes
C)23 minutes
D)36 minutes
E)45 minutes
A)15 minutes
B)18 minutes
C)23 minutes
D)36 minutes
E)45 minutes

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
33
The kinetics of the reaction ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -Calculate the value of k (ignore units).</strong> A)22 B)10 C)50 D)1.1 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -Calculate the value of k (ignore units).</strong> A)22 B)10 C)50 D)1.1 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-Calculate the value of k (ignore units).
A)22
B)10
C)50
D)1.1
E)none of these
![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -Calculate the value of k (ignore units).</strong> A)22 B)10 C)50 D)1.1 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![<strong>The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -Calculate the value of k (ignore units).</strong> A)22 B)10 C)50 D)1.1 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-Calculate the value of k (ignore units).
A)22
B)10
C)50
D)1.1
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
34
For a reaction: ![<strong>For a reaction: ,[A]<sub>0</sub> = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.</strong> A)5.7 M B)12 M C)0.68 M D)0.33 M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38d_93a6_27a8b3d513d1_TB6423_11.jpg) ,[A]0 = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.
,[A]0 = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.
A)5.7 M
B)12 M
C)0.68 M
D)0.33 M
E)none of these
![<strong>For a reaction: ,[A]<sub>0</sub> = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.</strong> A)5.7 M B)12 M C)0.68 M D)0.33 M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f8_f38d_93a6_27a8b3d513d1_TB6423_11.jpg) ,[A]0 = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.
,[A]0 = 6.0 M,and the first two half-lives are 56 and 28 minutes,respectively.Calculate [A] at t = 105.9 minutes.A)5.7 M
B)12 M
C)0.68 M
D)0.33 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
35
The concentration of O2 at t = 10.minutes is
A)2.0 10-4 mol/L
B)0.32 10-2 mol/L
C)0.16 10-2 mol/L
D)0.64 10-2 mol/L
E)none of these
A)2.0 10-4 mol/L
B)0.32 10-2 mol/L
C)0.16 10-2 mol/L
D)0.64 10-2 mol/L
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
36
The following data were obtained for the reaction 2A + B C where rate = [C]/ t
![<strong>The following data were obtained for the reaction 2A + B \to C where rate = \Delta [C]/ \Delta t What is the value of the rate constant?</strong> A)2.13 B)0.213 C)0.426 D)1.70 E)none of these](https://storage.examlex.com/TB6423/11efdc19_1f63_7791_8f65_29ef1e03f265_TB6423_00.jpg)
What is the value of the rate constant?
A)2.13
B)0.213
C)0.426
D)1.70
E)none of these
![<strong>The following data were obtained for the reaction 2A + B \to C where rate = \Delta [C]/ \Delta t What is the value of the rate constant?</strong> A)2.13 B)0.213 C)0.426 D)1.70 E)none of these](https://storage.examlex.com/TB6423/11efdc19_1f63_7791_8f65_29ef1e03f265_TB6423_00.jpg)
What is the value of the rate constant?
A)2.13
B)0.213
C)0.426
D)1.70
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
37
The reaction H2SeO3(aq)6I-(aq)+ 4H+(aq) 2I3-(aq)+ 3H2O(l)+ Se(s)was studied at 0°C by the method of initial rates:
![<strong>The reaction H<sub>2</sub>SeO<sub>3</sub>(aq)6I<sup>-</sup>(aq)+ 4H<sup>+</sup>(aq) \to 2I<sub>3</sub><sup>-</sup>(aq)+ 3H<sub>2</sub>O(l)+ Se(s)was studied at 0°C by the method of initial rates: -The rate law is</strong> A)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>] B)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>] C)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>]<sup>2</sup> D)Rate = k[H<sub>2</sub>SeO<sub>3</sub>]<sup>2</sup>[H<sup>+</sup>][I<sup>-</sup>] E)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>3</sup>](https://storage.examlex.com/TB6423/11efdc18_d91e_6c40_8f65_2376a13bb3f3_TB6423_00.jpg)
-The rate law is
A)Rate = k[H2SeO3][H+][I-]
B)Rate = k[H2SeO3][H+]2[I-]
C)Rate = k[H2SeO3][H+][I-]2
D)Rate = k[H2SeO3]2[H+][I-]
E)Rate = k[H2SeO3][H+]2[I-]3
![<strong>The reaction H<sub>2</sub>SeO<sub>3</sub>(aq)6I<sup>-</sup>(aq)+ 4H<sup>+</sup>(aq) \to 2I<sub>3</sub><sup>-</sup>(aq)+ 3H<sub>2</sub>O(l)+ Se(s)was studied at 0°C by the method of initial rates: -The rate law is</strong> A)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>] B)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>] C)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>][I<sup>-</sup>]<sup>2</sup> D)Rate = k[H<sub>2</sub>SeO<sub>3</sub>]<sup>2</sup>[H<sup>+</sup>][I<sup>-</sup>] E)Rate = k[H<sub>2</sub>SeO<sub>3</sub>][H<sup>+</sup>]<sup>2</sup>[I<sup>-</sup>]<sup>3</sup>](https://storage.examlex.com/TB6423/11efdc18_d91e_6c40_8f65_2376a13bb3f3_TB6423_00.jpg)
-The rate law is
A)Rate = k[H2SeO3][H+][I-]
B)Rate = k[H2SeO3][H+]2[I-]
C)Rate = k[H2SeO3][H+][I-]2
D)Rate = k[H2SeO3]2[H+][I-]
E)Rate = k[H2SeO3][H+]2[I-]3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
38
The concentration N2O5 at 100 minutes will be approximately
A)0.03 10-2 mol/L
B)0.06 10-2 mol/L
C)0.10 10-2 mol/L
D)0.01 10-2 mol/L
E)none of these
A)0.03 10-2 mol/L
B)0.06 10-2 mol/L
C)0.10 10-2 mol/L
D)0.01 10-2 mol/L
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
39
The following questions refer to the reaction between nitric oxide and hydrogen
2NO + H2 N2O + H2O

-What is the magnitude of the rate constant for this reaction?
A)0.66
B)4.2 10-3
C)870
D)1.9
E)300
2NO + H2 N2O + H2O

-What is the magnitude of the rate constant for this reaction?
A)0.66
B)4.2 10-3
C)870
D)1.9
E)300

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction 2A + 5B products is second order in A and first order in B.What is the rate law for this reaction?
A)rate = k[A]2[B]5
B)rate = k[A]1[B]2
C)rate = k[A]2[B]1
D)rate = k[A]5[B]2
E)rate = k[A]2/7[B]5/7
A)rate = k[A]2[B]5
B)rate = k[A]1[B]2
C)rate = k[A]2[B]1
D)rate = k[A]5[B]2
E)rate = k[A]2/7[B]5/7

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
41
The reaction ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> . s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 1.00 \times 10<sup>-</sup><sup>1</sup> M. The [NO] after 1.00 hour has passed is</strong> A)3.6 \times 10<sup>-</sup><sup>4 </sup>M B)9.9 \times 10<sup>-</sup><sup>3 </sup>M C)9.7 \times 10<sup>-</sup><sup>3 </sup>M D)1.0 \times 10<sup>-</sup><sup>3 </sup>M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_0510_93a6_373fdd2fc465_TB6423_11.jpg) exhibits the rate law
exhibits the rate law ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> . s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 1.00 \times 10<sup>-</sup><sup>1</sup> M. The [NO] after 1.00 hour has passed is</strong> A)3.6 \times 10<sup>-</sup><sup>4 </sup>M B)9.9 \times 10<sup>-</sup><sup>3 </sup>M C)9.7 \times 10<sup>-</sup><sup>3 </sup>M D)1.0 \times 10<sup>-</sup><sup>3 </sup>M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_0511_93a6_0b7a2668b832_TB6423_11.jpg) where k = 1.0 10-5 M-1 . s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 1.00 10-1 M. The [NO] after 1.00 hour has passed is
where k = 1.0 10-5 M-1 . s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 1.00 10-1 M. The [NO] after 1.00 hour has passed is
A)3.6 10-4 M
B)9.9 10-3 M
C)9.7 10-3 M
D)1.0 10-3 M
E)none of these
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> . s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 1.00 \times 10<sup>-</sup><sup>1</sup> M. The [NO] after 1.00 hour has passed is</strong> A)3.6 \times 10<sup>-</sup><sup>4 </sup>M B)9.9 \times 10<sup>-</sup><sup>3 </sup>M C)9.7 \times 10<sup>-</sup><sup>3 </sup>M D)1.0 \times 10<sup>-</sup><sup>3 </sup>M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_0510_93a6_373fdd2fc465_TB6423_11.jpg) exhibits the rate law
exhibits the rate law ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> . s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 1.00 \times 10<sup>-</sup><sup>1</sup> M. The [NO] after 1.00 hour has passed is</strong> A)3.6 \times 10<sup>-</sup><sup>4 </sup>M B)9.9 \times 10<sup>-</sup><sup>3 </sup>M C)9.7 \times 10<sup>-</sup><sup>3 </sup>M D)1.0 \times 10<sup>-</sup><sup>3 </sup>M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_0511_93a6_0b7a2668b832_TB6423_11.jpg) where k = 1.0 10-5 M-1 . s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 1.00 10-1 M. The [NO] after 1.00 hour has passed is
where k = 1.0 10-5 M-1 . s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 1.00 10-1 M. The [NO] after 1.00 hour has passed isA)3.6 10-4 M
B)9.9 10-3 M
C)9.7 10-3 M
D)1.0 10-3 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
42
The reaction A B + C is known to be zero order in A with a rate constant of 5.0 10-2 mol/L·s at 25°C.An experiment was run at 25°C where [A]0 = 1.0 10-3 M.
-The integrated rate law is
A)[A] = kt
B)[A] - [A]0 = kt
C)![<strong>The reaction A \to B + C is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L·s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M. -The integrated rate law is</strong> A)[A] = kt B)[A] - [A]<sub>0</sub> = kt C) D) E)[A]<sub>0</sub> - [A] = kt](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c1_93a6_930ef06d718d_TB6423_11.jpg)
D)![<strong>The reaction A \to B + C is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L·s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M. -The integrated rate law is</strong> A)[A] = kt B)[A] - [A]<sub>0</sub> = kt C) D) E)[A]<sub>0</sub> - [A] = kt](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c2_93a6_d3c2eed78e79_TB6423_11.jpg)
E)[A]0 - [A] = kt
-The integrated rate law is
A)[A] = kt
B)[A] - [A]0 = kt
C)
![<strong>The reaction A \to B + C is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L·s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M. -The integrated rate law is</strong> A)[A] = kt B)[A] - [A]<sub>0</sub> = kt C) D) E)[A]<sub>0</sub> - [A] = kt](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c1_93a6_930ef06d718d_TB6423_11.jpg)
D)
![<strong>The reaction A \to B + C is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L·s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M. -The integrated rate law is</strong> A)[A] = kt B)[A] - [A]<sub>0</sub> = kt C) D) E)[A]<sub>0</sub> - [A] = kt](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c2_93a6_d3c2eed78e79_TB6423_11.jpg)
E)[A]0 - [A] = kt

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
43
At a particular temperature,N2O5 decomposes according to a first-order rate law with a half-life of 3.0 s.If the initial concentration of N2O5 is 1.0 1016 molecules/cm3,what will be the concentration in molecules/cm3 after 16.1 s?
A)2.4 1014
B)3.3 101
C)1.0 1016
D)1.4 1014
E)2.3 10-1
A)2.4 1014
B)3.3 101
C)1.0 1016
D)1.4 1014
E)2.3 10-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
44
The reaction ![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 2.0 \times 10<sup>-</sup><sup>3</sup> M.After 5.0 minutes,the rate is</strong> A)5.0 \times 10<sup>-</sup><sup>2 </sup>mol/L·s B)2.5 \times 10<sup>-</sup><sup>2 </sup>mol/L·s C)1.3 \times 10<sup>-</sup><sup>2 </sup>mol/L·s D)2.0 \times 10<sup>-</sup><sup>3 </sup>mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c3_93a6_1d16ba24d7b5_TB6423_11.jpg) is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = 2.0 10-3 M.After 5.0 minutes,the rate is
is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = 2.0 10-3 M.After 5.0 minutes,the rate is
A)5.0 10-2 mol/L·s
B)2.5 10-2 mol/L·s
C)1.3 10-2 mol/L·s
D)2.0 10-3 mol/L·s
E)none of these
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = 2.0 \times 10<sup>-</sup><sup>3</sup> M.After 5.0 minutes,the rate is</strong> A)5.0 \times 10<sup>-</sup><sup>2 </sup>mol/L·s B)2.5 \times 10<sup>-</sup><sup>2 </sup>mol/L·s C)1.3 \times 10<sup>-</sup><sup>2 </sup>mol/L·s D)2.0 \times 10<sup>-</sup><sup>3 </sup>mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_68c3_93a6_1d16ba24d7b5_TB6423_11.jpg) is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = 2.0 10-3 M.After 5.0 minutes,the rate is
is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = 2.0 10-3 M.After 5.0 minutes,the rate isA)5.0 10-2 mol/L·s
B)2.5 10-2 mol/L·s
C)1.3 10-2 mol/L·s
D)2.0 10-3 mol/L·s
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
45
The reaction ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6ea_93a6_5db569d7b2bd_TB6423_11.jpg) exhibits the rate law
exhibits the rate law ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6eb_93a6_3937b73d17e5_TB6423_11.jpg) where k = 1.0 10-5 M-1 s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 0.11 M.What is one half-life for this experiment?
where k = 1.0 10-5 M-1 s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 0.11 M.What is one half-life for this experiment?
A)![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6ec_93a6_1bb0be28e809_TB6423_11.jpg) s
s
B)![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddfd_93a6_a51865d5b806_TB6423_11.jpg)
C)![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddfe_93a6_7bcf43cf1fe1_TB6423_11.jpg) s
s
D)![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddff_93a6_7327ccbf40bb_TB6423_11.jpg) s
s
E)none of these
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6ea_93a6_5db569d7b2bd_TB6423_11.jpg) exhibits the rate law
exhibits the rate law ![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6eb_93a6_3937b73d17e5_TB6423_11.jpg) where k = 1.0 10-5 M-1 s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 0.11 M.What is one half-life for this experiment?
where k = 1.0 10-5 M-1 s-1 at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]0)is 0.11 M.What is one half-life for this experiment?A)
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6ec_93a6_1bb0be28e809_TB6423_11.jpg) s
sB)
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddfd_93a6_a51865d5b806_TB6423_11.jpg)
C)
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddfe_93a6_7bcf43cf1fe1_TB6423_11.jpg) s
sD)
![<strong>The reaction exhibits the rate law where k = 1.0 \times 10<sup>-</sup><sup>5</sup> M<sup>-</sup><sup>1</sup> s<sup>-</sup><sup>1</sup> at 25°C.This reaction is run where the initial concentration of NOBr ([NOBr]<sub>0</sub>)is 0.11 M.What is one half-life for this experiment?</strong> A) s B) C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_ddff_93a6_7327ccbf40bb_TB6423_11.jpg) s
sE)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
46
A chemical reaction that is first order in X is observed to have a rate constant of 1.7 10-2s-1.If the initial concentration of X is 1.0 M,what is the concentration of X after 190 s?
A)25 M
B)0.68 M
C)0.24 M
D)0.96 M
E)0.040 M
A)25 M
B)0.68 M
C)0.24 M
D)0.96 M
E)0.040 M

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
47
The OH· radical disproportionates according to the elementary chemical reaction 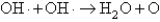 This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?
A)3.5 1011 s
B)2.6 101 s
C)3.8 10-2 s
D)7.7 10-14 s
E)1.9 10-2 s
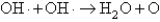 This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second order in OH·.The rate constant for the reaction is 2.0 10-12 cm3/molecules at room temperature.If the initial OH concentration is 1.3 1013 molecules/cm3,what is the first half-life for the reaction?A)3.5 1011 s
B)2.6 101 s
C)3.8 10-2 s
D)7.7 10-14 s
E)1.9 10-2 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the magnitude of the pseudo-rate constant (k')if the magnitude of X in the rate data is 0.00905.
A)4.3 10-3
B)1.2 10-2
C)0.86
D)0.31
E)1.81 10-3
A)4.3 10-3
B)1.2 10-2
C)0.86
D)0.31
E)1.81 10-3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
49
For the reaction A Products,successive half-lives are observed to be 10.0 min and 40.0 min.
-The reaction follows the integrated rate law
A)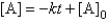
B)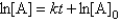
C)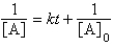
D)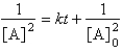
E)none of these
-The reaction follows the integrated rate law
A)
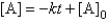
B)
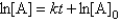
C)
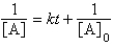
D)
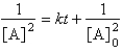
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
50
At a given temperature,a first-order reaction has a rate constant of 3.4 10-3 s-1.The time required for the reaction to be 44% completed is
A)4.0 min
B)1.2 min
C)20 min
D)2.8 min
E)19 min
A)4.0 min
B)1.2 min
C)20 min
D)2.8 min
E)19 min

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
51
For the reaction A Products,successive half-lives are observed to be 10.0 min and 40.0 min.
-The reaction![<strong>For the reaction A \to Products,successive half-lives are observed to be 10.0 min and 40.0 min. -The reaction is first order in N<sub>2</sub>O<sub>5</sub>.For this reaction at 45<sup>o</sup>C,the rate constant k = 1.0 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup>,where the rate law is defined as For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3 </sup>M),calculate [N<sub>2</sub>O<sub>5</sub>] after 3.5 \times 10<sup>5</sup> seconds.</strong> A)3.5 M B)1.0 \times 10<sup>-</sup><sup>3</sup> M C)3.0 \times 10<sup>-</sup><sup>5</sup> M D)0 M E)10 M](https://storage.examlex.com/TB6423/11eaa8f0_66fa_2c26_93a6_dbf7104cfd94_TB6423_11.jpg) is first order in N2O5.For this reaction at 45oC,the rate constant k = 1.0 10-5 s-1,where the rate law is defined as
is first order in N2O5.For this reaction at 45oC,the rate constant k = 1.0 10-5 s-1,where the rate law is defined as ![<strong>For the reaction A \to Products,successive half-lives are observed to be 10.0 min and 40.0 min. -The reaction is first order in N<sub>2</sub>O<sub>5</sub>.For this reaction at 45<sup>o</sup>C,the rate constant k = 1.0 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup>,where the rate law is defined as For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3 </sup>M),calculate [N<sub>2</sub>O<sub>5</sub>] after 3.5 \times 10<sup>5</sup> seconds.</strong> A)3.5 M B)1.0 \times 10<sup>-</sup><sup>3</sup> M C)3.0 \times 10<sup>-</sup><sup>5</sup> M D)0 M E)10 M](https://storage.examlex.com/TB6423/11eaa8f0_66fa_2c27_93a6_750651088578_TB6423_11.jpg) For a particular experiment ([N2O5]0 = 1.0 10-3 M),calculate [N2O5] after 3.5 105 seconds.
For a particular experiment ([N2O5]0 = 1.0 10-3 M),calculate [N2O5] after 3.5 105 seconds.
A)3.5 M
B)1.0 10-3 M
C)3.0 10-5 M
D)0 M
E)10 M
-The reaction
![<strong>For the reaction A \to Products,successive half-lives are observed to be 10.0 min and 40.0 min. -The reaction is first order in N<sub>2</sub>O<sub>5</sub>.For this reaction at 45<sup>o</sup>C,the rate constant k = 1.0 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup>,where the rate law is defined as For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3 </sup>M),calculate [N<sub>2</sub>O<sub>5</sub>] after 3.5 \times 10<sup>5</sup> seconds.</strong> A)3.5 M B)1.0 \times 10<sup>-</sup><sup>3</sup> M C)3.0 \times 10<sup>-</sup><sup>5</sup> M D)0 M E)10 M](https://storage.examlex.com/TB6423/11eaa8f0_66fa_2c26_93a6_dbf7104cfd94_TB6423_11.jpg) is first order in N2O5.For this reaction at 45oC,the rate constant k = 1.0 10-5 s-1,where the rate law is defined as
is first order in N2O5.For this reaction at 45oC,the rate constant k = 1.0 10-5 s-1,where the rate law is defined as ![<strong>For the reaction A \to Products,successive half-lives are observed to be 10.0 min and 40.0 min. -The reaction is first order in N<sub>2</sub>O<sub>5</sub>.For this reaction at 45<sup>o</sup>C,the rate constant k = 1.0 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup>,where the rate law is defined as For a particular experiment ([N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3 </sup>M),calculate [N<sub>2</sub>O<sub>5</sub>] after 3.5 \times 10<sup>5</sup> seconds.</strong> A)3.5 M B)1.0 \times 10<sup>-</sup><sup>3</sup> M C)3.0 \times 10<sup>-</sup><sup>5</sup> M D)0 M E)10 M](https://storage.examlex.com/TB6423/11eaa8f0_66fa_2c27_93a6_750651088578_TB6423_11.jpg) For a particular experiment ([N2O5]0 = 1.0 10-3 M),calculate [N2O5] after 3.5 105 seconds.
For a particular experiment ([N2O5]0 = 1.0 10-3 M),calculate [N2O5] after 3.5 105 seconds.A)3.5 M
B)1.0 10-3 M
C)3.0 10-5 M
D)0 M
E)10 M

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the reaction 3A + B + C D + E where the rate law is defined as ![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The concentration of C after 10.0 minutes is</strong> A)1.00 M B)1.10 \times 10<sup>-</sup><sup>5</sup> M C)0.330 M D)0.100 M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The concentration of C after 10.0 minutes is
A)1.00 M
B)1.10 10-5 M
C)0.330 M
D)0.100 M
E)none of these
![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The concentration of C after 10.0 minutes is</strong> A)1.00 M B)1.10 \times 10<sup>-</sup><sup>5</sup> M C)0.330 M D)0.100 M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The concentration of C after 10.0 minutes is
A)1.00 M
B)1.10 10-5 M
C)0.330 M
D)0.100 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
53
The reaction ![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd4_93a6_799aee19abe0_TB6423_11.jpg) is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 =
is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = ![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd5_93a6_a5aa1aba3371_TB6423_11.jpg) M.The half-life for the reaction is
M.The half-life for the reaction is
A)![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd6_93a6_d9d31c313310_TB6423_11.jpg) s
s
B)![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd7_93a6_25634a2c0d08_TB6423_11.jpg) s
s
C)![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6e8_93a6_83234f34a14a_TB6423_11.jpg) s
s
D)![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6e9_93a6_c9762cf570c4_TB6423_11.jpg) s
s
E)none of these
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd4_93a6_799aee19abe0_TB6423_11.jpg) is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 =
is known to be zero order in A with a rate constant of 5.0 10-2 mol/L s at 25°C.An experiment was run at 25°C where [A]0 = ![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd5_93a6_a5aa1aba3371_TB6423_11.jpg) M.The half-life for the reaction is
M.The half-life for the reaction isA)
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd6_93a6_d9d31c313310_TB6423_11.jpg) s
sB)
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_8fd7_93a6_25634a2c0d08_TB6423_11.jpg) s
sC)
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6e8_93a6_83234f34a14a_TB6423_11.jpg) s
sD)
![<strong>The reaction is known to be zero order in A with a rate constant of 5.0 \times 10<sup>-</sup><sup>2</sup> mol/L s at 25°C.An experiment was run at 25°C where [A]<sub>0</sub> = <sup> </sup>M.The half-life for the reaction is</strong> A) <sup> </sup>s B) <sup> </sup>s C) s D) <sup> </sup>s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66f9_b6e9_93a6_c9762cf570c4_TB6423_11.jpg) s
sE)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the reaction 3A + B + C D + E where the rate law is defined as ![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -After 3.00 minutes,[A] = 3.26 \times 10<sup>-</sup><sup>5</sup> M.The value of k is</strong> A)6.23 \times 10<sup>-</sup><sup>3</sup> L<sup>3</sup>/mol<sup>3</sup>·s B)3.26 \times 10<sup>-</sup><sup>5</sup> L<sup>3</sup>/mol<sup>3</sup>·s C)1.15 \times 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup>·s D)1.00 \times 10<sup>8</sup> L<sup>3</sup>/mol<sup>3</sup>·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-After 3.00 minutes,[A] = 3.26 10-5 M.The value of k is
A)6.23 10-3 L3/mol3·s
B)3.26 10-5 L3/mol3·s
C)1.15 102 L3/mol3·s
D)1.00 108 L3/mol3·s
E)none of these
![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -After 3.00 minutes,[A] = 3.26 \times 10<sup>-</sup><sup>5</sup> M.The value of k is</strong> A)6.23 \times 10<sup>-</sup><sup>3</sup> L<sup>3</sup>/mol<sup>3</sup>·s B)3.26 \times 10<sup>-</sup><sup>5</sup> L<sup>3</sup>/mol<sup>3</sup>·s C)1.15 \times 10<sup>2</sup> L<sup>3</sup>/mol<sup>3</sup>·s D)1.00 \times 10<sup>8</sup> L<sup>3</sup>/mol<sup>3</sup>·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-After 3.00 minutes,[A] = 3.26 10-5 M.The value of k is
A)6.23 10-3 L3/mol3·s
B)3.26 10-5 L3/mol3·s
C)1.15 102 L3/mol3·s
D)1.00 108 L3/mol3·s
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the reaction 3A + B + C D + E where the rate law is defined as ![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The concentration of A after 10.0 minutes is</strong> A)1.06 \times 10<sup>-</sup><sup>9</sup> M B)2.38 \times 10<sup>-</sup><sup>6</sup> M C)9.80 \times 10<sup>-</sup><sup>6</sup> M D)1.27 \times 10<sup>-</sup><sup>5</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The concentration of A after 10.0 minutes is
A)1.06 10-9 M
B)2.38 10-6 M
C)9.80 10-6 M
D)1.27 10-5 M
E)none of these
![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The concentration of A after 10.0 minutes is</strong> A)1.06 \times 10<sup>-</sup><sup>9</sup> M B)2.38 \times 10<sup>-</sup><sup>6</sup> M C)9.80 \times 10<sup>-</sup><sup>6</sup> M D)1.27 \times 10<sup>-</sup><sup>5</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The concentration of A after 10.0 minutes is
A)1.06 10-9 M
B)2.38 10-6 M
C)9.80 10-6 M
D)1.27 10-5 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
56
The reaction ![<strong>The reaction is second order in A.When [A]<sub>0</sub> = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).</strong> A)6.96 \times 10<sup>-</sup><sup>2</sup> B)5.57 \times 10<sup>-</sup><sup>4</sup> C)1.57 D)1.11 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_7a49_93a6_775d41f201df_TB6423_11.jpg) is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).
is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).
A)6.96 10-2
B)5.57 10-4
C)1.57
D)1.11
E)none of these
![<strong>The reaction is second order in A.When [A]<sub>0</sub> = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).</strong> A)6.96 \times 10<sup>-</sup><sup>2</sup> B)5.57 \times 10<sup>-</sup><sup>4</sup> C)1.57 D)1.11 E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_7a49_93a6_775d41f201df_TB6423_11.jpg) is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).
is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 35.9 minutes.Calculate the value of the rate constant (in L/min·mol).A)6.96 10-2
B)5.57 10-4
C)1.57
D)1.11
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
57
What is the concentration of B after 5 10-3 sec?
A)5.0 10-5 M
B)5.0 10-4 M
C)7.5 10-4 M
D)2.5 10-4 M
E)none of these
A)5.0 10-5 M
B)5.0 10-4 M
C)7.5 10-4 M
D)2.5 10-4 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction ![<strong>The reaction is second order in A.When [A]<sub>0</sub> = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.</strong> A)1.73 \times 10<sup>2</sup> min B)10.8 min C)2.16 \times 10<sup>4 </sup>min D)7.68 min E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_7a4a_93a6_3bb66ffa0d66_TB6423_11.jpg) is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.
is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.
A)1.73 102 min
B)10.8 min
C)2.16 104 min
D)7.68 min
E)none of these
![<strong>The reaction is second order in A.When [A]<sub>0</sub> = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.</strong> A)1.73 \times 10<sup>2</sup> min B)10.8 min C)2.16 \times 10<sup>4 </sup>min D)7.68 min E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_7a4a_93a6_3bb66ffa0d66_TB6423_11.jpg) is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.
is second order in A.When [A]0 = 0.100 M,the reaction is 20.0% complete in 43.2 minutes.Calculate the half-life for the reaction.A)1.73 102 min
B)10.8 min
C)2.16 104 min
D)7.68 min
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
59
A first-order reaction is 40.0% complete at the end of 48.1 minutes.What is the value of the rate constant (in min-1)?
A)1.90 10-2
B)1.06 10-2
C)94.2
D)52.5
E)none of these
A)1.90 10-2
B)1.06 10-2
C)94.2
D)52.5
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the reaction 3A + B + C D + E where the rate law is defined as ![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The half-life for this experiment is</strong> A)1.11 \times 10<sup>2</sup> s B)87.0 s C)6.03 \times 10<sup>-</sup><sup>3</sup> s D)117 s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.
An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The half-life for this experiment is
A)1.11 102 s
B)87.0 s
C)6.03 10-3 s
D)117 s
E)none of these
![<strong>Consider the reaction 3A + B + C \to D + E where the rate law is defined as . An experiment is carried out where [B]<sub>0</sub> = [C]<sub>0</sub> = 1.00 M and [A]<sub>0</sub> = 1.00 \times 10<sup>-</sup><sup>4</sup> M. -The half-life for this experiment is</strong> A)1.11 \times 10<sup>2</sup> s B)87.0 s C)6.03 \times 10<sup>-</sup><sup>3</sup> s D)117 s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_5338_93a6_f9c169f1857b_TB6423_11_TB6423_11_TB6423_11_TB6423_11.jpg) .
.An experiment is carried out where [B]0 = [C]0 = 1.00 M and [A]0 = 1.00 10-4 M.
-The half-life for this experiment is
A)1.11 102 s
B)87.0 s
C)6.03 10-3 s
D)117 s
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
61
Two isomers (A and B)of a given compound dimerize as follows:
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the concentration of A<sub>2</sub> after 3.0 minutes.</strong> A)2.8 \times 10<sup>-</sup><sup>22</sup> M B)6.9 \times 10<sup>-</sup><sup>3</sup> M C)3.1 \times 10<sup>-</sup><sup>3</sup> M D)1.6 \times 10<sup>-</sup><sup>3</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7e_93a6_ef2701f367bd_TB6423_11_TB6423_11.jpg)
Both processes are known to be second order in reactant,and k1 is known to be 0.25 L/mol·s at 25°C,where
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the concentration of A<sub>2</sub> after 3.0 minutes.</strong> A)2.8 \times 10<sup>-</sup><sup>22</sup> M B)6.9 \times 10<sup>-</sup><sup>3</sup> M C)3.1 \times 10<sup>-</sup><sup>3</sup> M D)1.6 \times 10<sup>-</sup><sup>3</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7f_93a6_833870defd7a_TB6423_11_TB6423_11.jpg)
In a particular experiment,A and B were placed in separate containers at 25°,where [A]0 = 1.0 10-2 M and [B]0 = 2.5 10-2 M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes.
-Calculate the concentration of A2 after 3.0 minutes.
A)2.8 10-22 M
B)6.9 10-3 M
C)3.1 10-3 M
D)1.6 10-3 M
E)none of these
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the concentration of A<sub>2</sub> after 3.0 minutes.</strong> A)2.8 \times 10<sup>-</sup><sup>22</sup> M B)6.9 \times 10<sup>-</sup><sup>3</sup> M C)3.1 \times 10<sup>-</sup><sup>3</sup> M D)1.6 \times 10<sup>-</sup><sup>3</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7e_93a6_ef2701f367bd_TB6423_11_TB6423_11.jpg)
Both processes are known to be second order in reactant,and k1 is known to be 0.25 L/mol·s at 25°C,where
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the concentration of A<sub>2</sub> after 3.0 minutes.</strong> A)2.8 \times 10<sup>-</sup><sup>22</sup> M B)6.9 \times 10<sup>-</sup><sup>3</sup> M C)3.1 \times 10<sup>-</sup><sup>3</sup> M D)1.6 \times 10<sup>-</sup><sup>3</sup> M E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7f_93a6_833870defd7a_TB6423_11_TB6423_11.jpg)
In a particular experiment,A and B were placed in separate containers at 25°,where [A]0 = 1.0 10-2 M and [B]0 = 2.5 10-2 M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes.
-Calculate the concentration of A2 after 3.0 minutes.
A)2.8 10-22 M
B)6.9 10-3 M
C)3.1 10-3 M
D)1.6 10-3 M
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
62
The rate law for a reaction is found to be Rate = k[A]2[B].Which of the following mechanisms gives this rate law?
I.A + B![<strong>The rate law for a reaction is found to be Rate = k[A]<sup>2</sup>[B].Which of the following mechanisms gives this rate law? I.A + B E (fast) E + B \to C + D (slow) II.A + B E (fast) E + A \to C + D (slow) III.A + A \to E (slow). E + B \to C + D (fast)</strong> A)I B)II C)III D)two of these E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_d9f0_93a6_2d00dda4c903_TB6423_11.jpg) E (fast)
E (fast)
E + B C + D (slow)
II.A + B![<strong>The rate law for a reaction is found to be Rate = k[A]<sup>2</sup>[B].Which of the following mechanisms gives this rate law? I.A + B E (fast) E + B \to C + D (slow) II.A + B E (fast) E + A \to C + D (slow) III.A + A \to E (slow). E + B \to C + D (fast)</strong> A)I B)II C)III D)two of these E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_d9f1_93a6_97991a33df82_TB6423_11.jpg) E (fast)
E (fast)
E + A C + D (slow)
III.A + A E (slow).
E + B C + D (fast)
A)I
B)II
C)III
D)two of these
E)none of these
I.A + B
![<strong>The rate law for a reaction is found to be Rate = k[A]<sup>2</sup>[B].Which of the following mechanisms gives this rate law? I.A + B E (fast) E + B \to C + D (slow) II.A + B E (fast) E + A \to C + D (slow) III.A + A \to E (slow). E + B \to C + D (fast)</strong> A)I B)II C)III D)two of these E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_d9f0_93a6_2d00dda4c903_TB6423_11.jpg) E (fast)
E (fast)E + B C + D (slow)
II.A + B
![<strong>The rate law for a reaction is found to be Rate = k[A]<sup>2</sup>[B].Which of the following mechanisms gives this rate law? I.A + B E (fast) E + B \to C + D (slow) II.A + B E (fast) E + A \to C + D (slow) III.A + A \to E (slow). E + B \to C + D (fast)</strong> A)I B)II C)III D)two of these E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_d9f1_93a6_97991a33df82_TB6423_11.jpg) E (fast)
E (fast)E + A C + D (slow)
III.A + A E (slow).
E + B C + D (fast)
A)I
B)II
C)III
D)two of these
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
63
A particular first-order reaction has a rate constant of 0.0107 s-1.What is the half-life for this reaction?
A)1.00 s
B)64.6 s
C)93.2 s
D)0.0155 s
E)0.0107 s
A)1.00 s
B)64.6 s
C)93.2 s
D)0.0155 s
E)0.0107 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
64
The experimental rate law for the decomposition of nitrous oxide (N2O)to N2 and O2 is Rate = k[N2O]2.Two mechanisms are proposed:
I.N2O N2 + O
N2O + O N2 + O2
II.2N2O![<strong>The experimental rate law for the decomposition of nitrous oxide (N<sub>2</sub>O)to N<sub>2</sub> and O<sub>2</sub> is Rate = k[N<sub>2</sub>O]<sup>2</sup>.Two mechanisms are proposed: I.N<sub>2</sub>O \to N<sub>2</sub> + O N<sub>2</sub>O + O \to N<sub>2</sub> + O<sub>2</sub> II.2N<sub>2</sub>O N<sub>4</sub>O<sub>2</sub> N<sub>4</sub>O<sub>2</sub> \to 2N<sub>2</sub> + O<sub>2</sub> Which of the following could be a correct mechanism?</strong> A)Mechanism I,with the first step as the rate-determining step. B)Mechanism I,with the second step as the rate-determining step as long as the first step is a fast equilibrium step. C)Mechanism II,with the second step as the rate-determining step if the first step is a fast equilibrium step. D)None of the choices (A-C)could be correct. E)At least two of the above choices (A-C)could be correct.](https://storage.examlex.com/TB6423/11eaa8f0_66fc_0102_93a6_595a1f94c072_TB6423_11.jpg) N4O2
N4O2
N4O2 2N2 + O2
Which of the following could be a correct mechanism?
A)Mechanism I,with the first step as the rate-determining step.
B)Mechanism I,with the second step as the rate-determining step as long as the first step is a fast equilibrium step.
C)Mechanism II,with the second step as the rate-determining step if the first step is a fast equilibrium step.
D)None of the choices (A-C)could be correct.
E)At least two of the above choices (A-C)could be correct.
I.N2O N2 + O
N2O + O N2 + O2
II.2N2O
![<strong>The experimental rate law for the decomposition of nitrous oxide (N<sub>2</sub>O)to N<sub>2</sub> and O<sub>2</sub> is Rate = k[N<sub>2</sub>O]<sup>2</sup>.Two mechanisms are proposed: I.N<sub>2</sub>O \to N<sub>2</sub> + O N<sub>2</sub>O + O \to N<sub>2</sub> + O<sub>2</sub> II.2N<sub>2</sub>O N<sub>4</sub>O<sub>2</sub> N<sub>4</sub>O<sub>2</sub> \to 2N<sub>2</sub> + O<sub>2</sub> Which of the following could be a correct mechanism?</strong> A)Mechanism I,with the first step as the rate-determining step. B)Mechanism I,with the second step as the rate-determining step as long as the first step is a fast equilibrium step. C)Mechanism II,with the second step as the rate-determining step if the first step is a fast equilibrium step. D)None of the choices (A-C)could be correct. E)At least two of the above choices (A-C)could be correct.](https://storage.examlex.com/TB6423/11eaa8f0_66fc_0102_93a6_595a1f94c072_TB6423_11.jpg) N4O2
N4O2N4O2 2N2 + O2
Which of the following could be a correct mechanism?
A)Mechanism I,with the first step as the rate-determining step.
B)Mechanism I,with the second step as the rate-determining step as long as the first step is a fast equilibrium step.
C)Mechanism II,with the second step as the rate-determining step if the first step is a fast equilibrium step.
D)None of the choices (A-C)could be correct.
E)At least two of the above choices (A-C)could be correct.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
65
The radioactive nuclide  undergoes first-order decay with a half-life of 9.31 min.If a quantity of
undergoes first-order decay with a half-life of 9.31 min.If a quantity of 
Is produced,what fraction remains after 82.5 seconds?
A)0.113
B)0.00215
C)0.148
D)0.903
E)0.0973
 undergoes first-order decay with a half-life of 9.31 min.If a quantity of
undergoes first-order decay with a half-life of 9.31 min.If a quantity of 
Is produced,what fraction remains after 82.5 seconds?
A)0.113
B)0.00215
C)0.148
D)0.903
E)0.0973

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
66
Determine the molecularity of the following elementary reaction: O3 O2 + O.
A)unimolecular
B)bimolecular
C)termolecular
D)quadmolecular
E)molecularity cannot be determined
A)unimolecular
B)bimolecular
C)termolecular
D)quadmolecular
E)molecularity cannot be determined

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
67
Of what use is it to find a rate law for a reaction?
A)We can use the rate law to directly determine coefficients in the balanced equation.
B)From the rate law we can evaluate potential reaction mechanisms.
C)The rate law gives us a good indication of the thermodynamic stability of the products.
D)The rate law can lead us to determine the equilibrium constant for the reaction.
E)None of these.
A)We can use the rate law to directly determine coefficients in the balanced equation.
B)From the rate law we can evaluate potential reaction mechanisms.
C)The rate law gives us a good indication of the thermodynamic stability of the products.
D)The rate law can lead us to determine the equilibrium constant for the reaction.
E)None of these.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
68
The reaction A products is first order.If the initial concentration of A is 0.528 M and,after 16.3 seconds have elapsed,the concentration of A has fallen to 0.0122 M,what is the rate constant of the reaction?
A)0.231 s-1
B)0.0425 s-1
C)0.0316 s-1
D)4.91 s-1
E)0.0613 s-1
A)0.231 s-1
B)0.0425 s-1
C)0.0316 s-1
D)4.91 s-1
E)0.0613 s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction A products is second order.If the initial concentration of A is 0.436 M and,after 52.8 seconds have elapsed,the concentration of A has fallen to 0.0476 M,what is the rate constant of the reaction?
A)0.0419 M-1 s-1
B)0.354 M-1 s-1
C)0.0131 M-1 s-1
D)0.00736 M-1 s-1
E)0.0189 M-1 s-1
A)0.0419 M-1 s-1
B)0.354 M-1 s-1
C)0.0131 M-1 s-1
D)0.00736 M-1 s-1
E)0.0189 M-1 s-1

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
70
The decomposition of N2O5(g)to NO2(g)and O2(g)obeys first-order kinetics.Assuming the form of the rate law is: ![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1692_93a6_45218845056e_TB6423_11.jpg)
Where k = 3.4 10-5 s-1 at 25°C,what is the initial rate of reaction at 25°C where [N2O5]0 = 8.2 10-2 M?
A)![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da3_93a6_7b5708d4ccf1_TB6423_11.jpg) mol/L·s
mol/L·s
B)![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da4_93a6_ade05276a406_TB6423_11.jpg) mol/L·s
mol/L·s
C)![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da5_93a6_e9fc09ca3539_TB6423_11.jpg) mol/L·s
mol/L·s
D)![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da6_93a6_7be9bf06ac01_TB6423_11.jpg) mol/L·s
mol/L·s
E)none of these
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1692_93a6_45218845056e_TB6423_11.jpg)
Where k = 3.4 10-5 s-1 at 25°C,what is the initial rate of reaction at 25°C where [N2O5]0 = 8.2 10-2 M?
A)
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da3_93a6_7b5708d4ccf1_TB6423_11.jpg) mol/L·s
mol/L·sB)
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da4_93a6_ade05276a406_TB6423_11.jpg) mol/L·s
mol/L·sC)
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da5_93a6_e9fc09ca3539_TB6423_11.jpg) mol/L·s
mol/L·sD)
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub>(g)to NO<sub>2</sub>(g)and O<sub>2</sub>(g)obeys first-order kinetics.Assuming the form of the rate law is: <sup> </sup> <sup> </sup> Where k = 3.4 \times 10<sup>-</sup><sup>5</sup> s<sup>-</sup><sup>1</sup> at 25°C,what is the initial rate of reaction at 25°C where [N<sub>2</sub>O<sub>5</sub>]<sub>0</sub> = 8.2 \times 10<sup>-</sup><sup>2</sup> M?</strong> A) mol/L·s B) mol/L·s C) <sup> </sup>mol/L·s D) mol/L·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_3da6_93a6_7be9bf06ac01_TB6423_11.jpg) mol/L·s
mol/L·sE)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
71
If the reaction 2HI H2 + I2 is second order,which of the following will yield a linear plot?
A)log [HI] vs time
B)1/[HI] vs time
C)[HI] vs time
D)ln [HI] vs time
E)None of these.
A)log [HI] vs time
B)1/[HI] vs time
C)[HI] vs time
D)ln [HI] vs time
E)None of these.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
72
The elementary chemical reaction O + ClO Cl + O2 is made pseudo-first order in oxygen atoms by using a large excess of ClO radicals.The rate constant for the reaction is 2.7 cm3/molecule.s.If the initial concentration of ClO is 1.0 1011 molecules/cm3,how long will it take for the oxygen atoms to decrease to 10.% of their initial concentration?
A)1.2 s
B)0.039 s
C)0.26 s
D)0.85 s
E)2.6 s
A)1.2 s
B)0.039 s
C)0.26 s
D)0.85 s
E)2.6 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
73
Two isomers (A and B)of a given compound dimerize as follows:
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7e_93a6_ef2701f367bd_TB6423_11_TB6423_11.jpg)
Both processes are known to be second order in reactant,and k1 is known to be 0.25 L/mol·s at 25°C,where
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7f_93a6_833870defd7a_TB6423_11_TB6423_11.jpg)
In a particular experiment,A and B were placed in separate containers at 25°,where [A]0 = 1.0 10-2 M and [B]0 = 2.5 10-2 M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes.
-Calculate the value of![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1690_93a6_c38983a9d3ff_TB6423_11.jpg) where
where
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1691_93a6_4b1dac189960_TB6423_11.jpg)
A)2.2 L/mol·s
B)0.75 L/mol·s
C)1.9 L/mol·s
D)0.21 L/mol·s
E)none of these
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7e_93a6_ef2701f367bd_TB6423_11_TB6423_11.jpg)
Both processes are known to be second order in reactant,and k1 is known to be 0.25 L/mol·s at 25°C,where
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fa_ef7f_93a6_833870defd7a_TB6423_11_TB6423_11.jpg)
In a particular experiment,A and B were placed in separate containers at 25°,where [A]0 = 1.0 10-2 M and [B]0 = 2.5 10-2 M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes.
-Calculate the value of
![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1690_93a6_c38983a9d3ff_TB6423_11.jpg) where
where ![<strong>Two isomers (A and B)of a given compound dimerize as follows: Both processes are known to be second order in reactant,and k<sub>1 </sub>is known to be 0.25 L/mol·s at 25°C,where <sup> </sup> In a particular experiment,A and B were placed in separate containers at 25°,where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>2</sup> M and [B]<sub>0</sub> = 2.5 \times 10<sup>-</sup><sup>2</sup> M.It was found that [A] = 3[B] after the reactions progressed for 3.0 minutes. -Calculate the value of where <sup> </sup> <sup> </sup> <sup> </sup></strong> A)2.2 L/mol·s B)0.75 L/mol·s C)1.9 L/mol·s D)0.21 L/mol·s E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fb_1691_93a6_4b1dac189960_TB6423_11.jpg)
A)2.2 L/mol·s
B)0.75 L/mol·s
C)1.9 L/mol·s
D)0.21 L/mol·s
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the reaction 2O3(g) 3O2(g).The following mechanism is proposed: O3 ![<strong>Consider the reaction 2O<sub>3</sub>(g) \to 3O<sub>2</sub>(g).The following mechanism is proposed: O<sub>3</sub> O<sub>2</sub> + O O<sub>3</sub> + O \to 2O<sub>2</sub> If we assume the second step of the mechanism is the rate determining step and the first step is a fast equilibrium step,which of the following rate laws is predicted by this mechanism?</strong> A)rate = k[O<sub>3</sub>] B)rate = k[O<sub>3</sub>]<sup>2</sup>[O<sub>2</sub>] C)rate = k[O<sub>3</sub>]<sup>2</sup>[O<sub>2</sub>]<sup>-</sup><sup>1</sup> D)rate = k[O<sub>3</sub>]<sup>2</sup> E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fc_2813_93a6_4df398e411e2_TB6423_11.jpg) O2 + O
O2 + O
O3 + O 2O2
If we assume the second step of the mechanism is the rate determining step and the first step is a fast equilibrium step,which of the following rate laws is predicted by this mechanism?
A)rate = k[O3]
B)rate = k[O3]2[O2]
C)rate = k[O3]2[O2]-1
D)rate = k[O3]2
E)none of these
![<strong>Consider the reaction 2O<sub>3</sub>(g) \to 3O<sub>2</sub>(g).The following mechanism is proposed: O<sub>3</sub> O<sub>2</sub> + O O<sub>3</sub> + O \to 2O<sub>2</sub> If we assume the second step of the mechanism is the rate determining step and the first step is a fast equilibrium step,which of the following rate laws is predicted by this mechanism?</strong> A)rate = k[O<sub>3</sub>] B)rate = k[O<sub>3</sub>]<sup>2</sup>[O<sub>2</sub>] C)rate = k[O<sub>3</sub>]<sup>2</sup>[O<sub>2</sub>]<sup>-</sup><sup>1</sup> D)rate = k[O<sub>3</sub>]<sup>2</sup> E)none of these](https://storage.examlex.com/TB6423/11eaa8f0_66fc_2813_93a6_4df398e411e2_TB6423_11.jpg) O2 + O
O2 + OO3 + O 2O2
If we assume the second step of the mechanism is the rate determining step and the first step is a fast equilibrium step,which of the following rate laws is predicted by this mechanism?
A)rate = k[O3]
B)rate = k[O3]2[O2]
C)rate = k[O3]2[O2]-1
D)rate = k[O3]2
E)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
75
The decomposition of ozone may occur through the two-step mechanism shown: 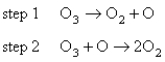 The oxygen atom is considered to be a(n)
The oxygen atom is considered to be a(n)
A)reactant
B)product
C)catalyst
D)reaction intermediate
E)activated complex
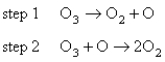 The oxygen atom is considered to be a(n)
The oxygen atom is considered to be a(n)A)reactant
B)product
C)catalyst
D)reaction intermediate
E)activated complex

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction ![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bcc_93a6_5f30d36235d9_TB6423_11.jpg) obeys the rate law:
obeys the rate law: ![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bcd_93a6_755131e7040c_TB6423_11.jpg) at 500.K. If the initial concentration of NO2 is 1.00 M,how long will it take for the [NO2] to decrease to 35.8% of its initial value?
at 500.K. If the initial concentration of NO2 is 1.00 M,how long will it take for the [NO2] to decrease to 35.8% of its initial value?
A)45.9 s
B)73 s
C)128 s
D)![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bce_93a6_e5a3da7a77ef_TB6423_11.jpg) s
s
E)cannot be determined from this data
![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bcc_93a6_5f30d36235d9_TB6423_11.jpg) obeys the rate law:
obeys the rate law: ![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bcd_93a6_755131e7040c_TB6423_11.jpg) at 500.K. If the initial concentration of NO2 is 1.00 M,how long will it take for the [NO2] to decrease to 35.8% of its initial value?
at 500.K. If the initial concentration of NO2 is 1.00 M,how long will it take for the [NO2] to decrease to 35.8% of its initial value?A)45.9 s
B)73 s
C)128 s
D)
![<strong>The reaction obeys the rate law: at 500.K. If the initial concentration of NO<sub>2</sub> is 1.00 M,how long will it take for the [NO<sub>2</sub>] to decrease to 35.8% of its initial value?</strong> A)45.9 s B)73 s C)128 s D) s E)cannot be determined from this data](https://storage.examlex.com/TB6423/11eaa8f0_66fb_8bce_93a6_e5a3da7a77ef_TB6423_11.jpg) s
sE)cannot be determined from this data

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
77
The decomposition of N2O5(g)to NO2(g)and O2(g)obeys first-order kinetics.Assuming the form of the rate law is: 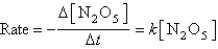
Where k = 5.4 10-5 s-1 at 25°C,what is the half-life for the reaction described?
A)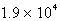 s
s
B)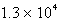 s
s
C)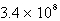 s
s
D)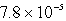 s
s
E)none of these
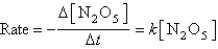
Where k = 5.4 10-5 s-1 at 25°C,what is the half-life for the reaction described?
A)
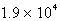 s
sB)
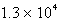 s
sC)
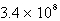 s
sD)
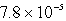 s
sE)none of these

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
78
In 6 M HCl,the complex ion Ru(NH3)63+ decomposes to a variety of products.The reaction is first order in Ru(NH3)63+ and has a half-life of 14 hours at 25°C.Under these conditions,how long will it take for the [Ru(NH3)63+] to decrease to 23.5% of its initial value?
A)5.4 hours
B)9.7 hours
C)3.3 hours
D)14 hours
E)29 hours
A)5.4 hours
B)9.7 hours
C)3.3 hours
D)14 hours
E)29 hours

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
79
The reaction 3NO N2O + NO2 is found to obey the rate law,Rate = k[NO]2.If the first half-life of the reaction is found to be 2.0 s,what is the length of the fourth half-life?
A)2.0 s
B)4.0 s
C)8.0 s
D)12.0 s
E)16.0 s
A)2.0 s
B)4.0 s
C)8.0 s
D)12.0 s
E)16.0 s

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
80
Consider a reaction of the type aA Products in which the rate law is found to be rate = k[A]3 (yes,a termolecular reaction is improbable but possible).If the first half-life of the reaction is found to be 40 seconds,what is the time for the second half-life?
A)10 seconds
B)20 seconds
C)80 seconds
D)160 seconds
E)320 seconds
A)10 seconds
B)20 seconds
C)80 seconds
D)160 seconds
E)320 seconds

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck


