Deck 17: Spontaneity, entropy, and Free Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 17: Spontaneity, entropy, and Free Energy
1
Consider the following processes:
I.condensation of a liquid
IIincreasing the volume of 1.0 mol of an ideal gas at constant temperature
III.dissolving sugar in water
IV.heating 1.0 mol of an ideal gas at constant volume
For how many of these is S positive?
A)0
B)1
C)2
D)3
E)4
I.condensation of a liquid
IIincreasing the volume of 1.0 mol of an ideal gas at constant temperature
III.dissolving sugar in water
IV.heating 1.0 mol of an ideal gas at constant volume
For how many of these is S positive?
A)0
B)1
C)2
D)3
E)4
3
2
Which of the following statements is true?
A)The total energy and entropy of the universe are both increasing.
B)The total energy of the universe is increasing,but the entropy is constant.
C)The total energy of the universe increases,while the entropy decreases.
D)The total energy of the universe is constant,but the entropy is increasing.
E)None of these.
A)The total energy and entropy of the universe are both increasing.
B)The total energy of the universe is increasing,but the entropy is constant.
C)The total energy of the universe increases,while the entropy decreases.
D)The total energy of the universe is constant,but the entropy is increasing.
E)None of these.
The total energy of the universe is constant,but the entropy is increasing.
3
Which of the following shows a decrease in entropy?
A)precipitation
B)gaseous reactants forming a liquid
C)a burning piece of wood
D)melting ice
E)two of these
A)precipitation
B)gaseous reactants forming a liquid
C)a burning piece of wood
D)melting ice
E)two of these
two of these
4
Assume that the enthalpy of fusion of ice is 6020 J/mol and does not vary appreciably over the temperature range 270-290 K.If one mole of ice at 0°C is melted by heat supplied from surroundings at 276 K,what is the entropy change in the surroundings,in J/K?
A)22.1
B)21.8
C)0.0
D)-21.8
E)-22.1
A)22.1
B)21.8
C)0.0
D)-21.8
E)-22.1

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following result(s)in an increase in the entropy of the system?
I.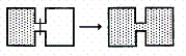
II.
Br2(g) Br2(l)
III.
NaBr(s) Na+(aq)+ Br-(aq)
IV.
O2(298 K) O2(373 K)
V.
NH3(1 atm,298 K) NH3(3 atm,298 K)
A)I
B)II,V
C)I,III,IV
D)I,II,III,IV
E)I,II,III,V
I.

II.
Br2(g) Br2(l)
III.
NaBr(s) Na+(aq)+ Br-(aq)
IV.
O2(298 K) O2(373 K)
V.
NH3(1 atm,298 K) NH3(3 atm,298 K)
A)I
B)II,V
C)I,III,IV
D)I,II,III,IV
E)I,II,III,V

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
The heat of vaporization for 1.0 mole of water at 100.°C and 1.0 atm is 40.56 kJ/mol.Calculate S for the process H2O(l) H2O(g)at 100.°C.
A)109 J/K mol
B)-109 J/K mol
C)406 J/K mol
D)-406 J/K mol
E)none of these
A)109 J/K mol
B)-109 J/K mol
C)406 J/K mol
D)-406 J/K mol
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
A two-bulbed flask contains 5 particles.What is the probability of finding all 5 particles on the left side?
A)2.50%
B)2.24%
C)3.13%
D)0.20%
E)6.25%
A)2.50%
B)2.24%
C)3.13%
D)0.20%
E)6.25%

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
Ten identical coins are shaken vigorously in a cup and then poured out onto a table top.Which of the following distributions has the highest probability of occurrence? (T = Tails,H = Heads)
A)T10H0
B)T8H2
C)T7H3
D)T5H5
E)T4H6
A)T10H0
B)T8H2
C)T7H3
D)T5H5
E)T4H6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
A change of state that occurs in a system is accompanied by 52.3 kJ of heat,which is transferred to the surroundings at a constant pressure and a constant temperature of 300.K.For this process Ssurr is:
A)52.3 kJ/K
B)-52.3 kJ/K
C)-174 J/K
D)174 J/K
E)248 kJ/K
A)52.3 kJ/K
B)-52.3 kJ/K
C)-174 J/K
D)174 J/K
E)248 kJ/K

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
A 100-mL sample of water is placed in a coffee cup calorimeter.When 1.0 g of an ionic solid is added,the temperature decreases from 21.5°C to 20.8°C as the solid dissolves.For the dissolving of the solid
A)( H < 0)
B)( Suniv > 0)
C)( Ssys< 0)
D)( Ssurr > 0)
E)none of these
A)( H < 0)
B)( Suniv > 0)
C)( Ssys< 0)
D)( Ssurr > 0)
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
If two pyramid-shaped dice (with numbers 1 through 4 on the sides)were tossed,which outcome has the highest entropy?
A)The sum of the dice is 3.
B)The sum of the dice is 4.
C)The sum of the dice is 5.
D)The sum of the dice is 6.
E)The sum of the dice is 7.
A)The sum of the dice is 3.
B)The sum of the dice is 4.
C)The sum of the dice is 5.
D)The sum of the dice is 6.
E)The sum of the dice is 7.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
At 1 atm,liquid water is heated above 100°C.
- Ssurr for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)
- Ssurr for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is true for exothermic processes?
A)( Ssurr < 0)
B)( Ssurr) = - H/T
C)( Ssurr = 0)
D)( Ssurr > 0)
E)two of these
A)( Ssurr < 0)
B)( Ssurr) = - H/T
C)( Ssurr = 0)
D)( Ssurr > 0)
E)two of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
Ssurr is _______ for exothermic reactions and ______ for endothermic reactions.
A)favorable,unfavorable
B)unfavorable,favorable
C)favorable,favorable
D)unfavorable,unfavorable
E)cannot tell
A)favorable,unfavorable
B)unfavorable,favorable
C)favorable,favorable
D)unfavorable,unfavorable
E)cannot tell

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
The enthalpy of vaporization of ammonia is 23.35 kJ/mol at its boiling point (-33.4°C).Calculate the value of Ssurr when 1.00 mole of ammonia is vaporized at -33.4°C and 1.00 atm.
A)0
B)-6.99 102 J/K mol
C)9.74 101 J/K mol
D)-9.74 101 J/K mol
E)6.99 102 J/K mol
A)0
B)-6.99 102 J/K mol
C)9.74 101 J/K mol
D)-9.74 101 J/K mol
E)6.99 102 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
At 1 atm,liquid water is heated above 100°C.
- Ssys for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)
- Ssys for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
For which process is S negative?
A)evaporation of 1 mol of CCl4(l)
B)mixing 5 mL ethanol with 25 mL water
C)compressing 1 mol Ne at constant temperature from 1.5 L to 0.5 L
D)raising the temperature of 100 g Cu from 275 K to 295 K
E)grinding a large crystal of KCl to powder
A)evaporation of 1 mol of CCl4(l)
B)mixing 5 mL ethanol with 25 mL water
C)compressing 1 mol Ne at constant temperature from 1.5 L to 0.5 L
D)raising the temperature of 100 g Cu from 275 K to 295 K
E)grinding a large crystal of KCl to powder

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
If the change in entropy of the surroundings for a process at 431 K and constant pressure is -326 J/K,what is the heat flow absorbed by for the system?
A)326 kJ
B)1.32 kJ
C)-141 kJ
D)105 kJ
E)141 kJ
A)326 kJ
B)1.32 kJ
C)-141 kJ
D)105 kJ
E)141 kJ

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
The second law of thermodynamics states that:
A)The entropy of a perfect crystal is zero at 0 K.
B)The entropy of the universe is constant.
C)The energy of the universe is increasing.
D)The entropy of the universe is increasing.
E)The energy of the universe is constant.
A)The entropy of a perfect crystal is zero at 0 K.
B)The entropy of the universe is constant.
C)The energy of the universe is increasing.
D)The entropy of the universe is increasing.
E)The energy of the universe is constant.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
A chemical reaction is most likely to be spontaneous if it is accompanied by
A)increasing energy and increasing entropy
B)lowering energy and increasing entropy
C)increasing energy and decreasing entropy
D)lowering energy and decreasing entropy
E)none of these (A-D)
A)increasing energy and increasing entropy
B)lowering energy and increasing entropy
C)increasing energy and decreasing entropy
D)lowering energy and decreasing entropy
E)none of these (A-D)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
Substance X has a heat of vaporization of 58.4 kJ/mol at its normal boiling point (423°C).For the process X(l) X(g)at 1 atm and 423°C calculate the value of Ssurr.
A)0
B)83.9 J/K mol
C)138 J/K mol
D)-83.9 J/K mol
E)-138 J/K mol
A)0
B)83.9 J/K mol
C)138 J/K mol
D)-83.9 J/K mol
E)-138 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
Given that Hvap is 58.2 kJ/mol,and the boiling point is 83.4°C,1 atm,if one mole of this substance is vaporized at 1 atm,calculate Ssurr.
A)-163 J/K mol
B)163 J/K mol
C)698 J/K mol
D)-698 J/K mol
E)0
A)-163 J/K mol
B)163 J/K mol
C)698 J/K mol
D)-698 J/K mol
E)0

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
Substance X has a heat of vaporization of 55.9 kJ/mol at its normal boiling point (423°C).For the process X(l) X(g)at 1 atm and 423°C calculate the value of Suniv.
A)0
B)80.3 J/K mol
C)132 J/K mol
D)-80.3 J/K mol
E)-132 J/K mol
A)0
B)80.3 J/K mol
C)132 J/K mol
D)-80.3 J/K mol
E)-132 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
At 1 atm,liquid water is heated above 100°C.
- Suniv for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)
- Suniv for this process is
A)greater than zero
B)less than zero
C)equal to zero
D)more information needed to answer this question
E)none of these (A-D)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
Substance X has a heat of vaporization of 64.4 kJ/mol at its normal boiling point (423°C).For the process X(l) X(g)at 1 atm and 423°C calculate the value of G.
A)0 J
B)92.5 J
C)152 J
D)-92.5 J
E)-152 J
A)0 J
B)92.5 J
C)152 J
D)-92.5 J
E)-152 J

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
The enthalpy of vaporization of ammonia is 23.35 kJ/mol at its boiling point (-33.4°C).Calculate the value of S when 1.00 mole of ammonia is vaporized at -33.4°C and 1.00 atm.
A)0
B)-6.99 102 J/K mol
C)9.74 101 J/K mol
D)-9.74 101 J/K mol
E)6.99 102 J/K mol
A)0
B)-6.99 102 J/K mol
C)9.74 101 J/K mol
D)-9.74 101 J/K mol
E)6.99 102 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
For the vaporization of a liquid at a given pressure:
A)( G) is positive at all temperatures.
B)( G) is negative at all temperatures.
C)( G) is positive at low temperatures,but negative at high temperatures (and zero at some temperature).
D)( G) is negative at low temperatures,but positive at high temperatures (and zero at some temperature).
E)None of these (A-D).
A)( G) is positive at all temperatures.
B)( G) is negative at all temperatures.
C)( G) is positive at low temperatures,but negative at high temperatures (and zero at some temperature).
D)( G) is negative at low temperatures,but positive at high temperatures (and zero at some temperature).
E)None of these (A-D).

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
S is _______ for exothermic reactions and ______ for endothermic reactions.
A)favorable,unfavorable
B)unfavorable,favorable
C)favorable,favorable
D)unfavorable,unfavorable
E)cannot tell
A)favorable,unfavorable
B)unfavorable,favorable
C)favorable,favorable
D)unfavorable,unfavorable
E)cannot tell

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
For any given process, Ssurr and Ssys have opposite signs.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
Given that Hvap is 60.3 kJ/mol,and the boiling point is 83.4°C,1 atm,if one mole of this substance is vaporized at 1 atm,calculate S.
A)-169 J/K mol
B)169 J/K mol
C)723 J/K mol
D)-723 J/K mol
E)0
A)-169 J/K mol
B)169 J/K mol
C)723 J/K mol
D)-723 J/K mol
E)0

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
For the process CHCl3(s) CHCl3(l), H° = 9.19 kJ/mol and S° = 43.9 J/mol/K.What is the melting point of chloroform?
A)-64 °C
B)209 °C
C)130 °C
D)64 °C
E)-130 °C
A)-64 °C
B)209 °C
C)130 °C
D)64 °C
E)-130 °C

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
Consider two perfectly insulated vessels.Vessel #1 initially contains an ice cube at 0°C and water at 0°C.Vessel #2 initially contains an ice cube at 0°C and a saltwater solution at 0°C.In each vessel,consider the "system" to be the ice,and the "surroundings" to be the liquid.
-Determine the sign of Ssys, Ssurr,and Suniv for the contents of Vessel #1.
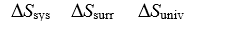
A)0 0 0
B)+ - 0
C)+ + +
D)+ - +
E)+ 0 +
-Determine the sign of Ssys, Ssurr,and Suniv for the contents of Vessel #1.
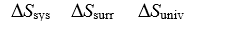
A)0 0 0
B)+ - 0
C)+ + +
D)+ - +
E)+ 0 +

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
H° is zero for a chemical reaction at constant temperature.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
Consider two perfectly insulated vessels.Vessel #1 initially contains an ice cube at 0°C and water at 0°C.Vessel #2 initially contains an ice cube at 0°C and a saltwater solution at 0°C.In each vessel,consider the "system" to be the ice,and the "surroundings" to be the liquid.
-Determine the sign of Ssys, Ssurr,and Suniv for the system (ice/saltwater)in Vessel #2.
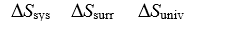
A)0 0 0
B)+ - 0
C)+ + +
D)+ - +
E)+ 0 +
-Determine the sign of Ssys, Ssurr,and Suniv for the system (ice/saltwater)in Vessel #2.
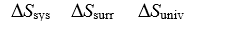
A)0 0 0
B)+ - 0
C)+ + +
D)+ - +
E)+ 0 +

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
The melting point of water is 0°C at 1 atm pressure because under these conditions:
A)( S) for the process H2O(s) H2O(l)is positive.
B)( S) and Ssurr for the process H2O(s) H2O(l)are both positive.
C)( S) and Ssurr for the process H2O(s) H2O(l)are equal in magnitude and opposite in sign.
D)( G) is positive for the process H2O(s) H2O(l).
E)None of these is correct.
A)( S) for the process H2O(s) H2O(l)is positive.
B)( S) and Ssurr for the process H2O(s) H2O(l)are both positive.
C)( S) and Ssurr for the process H2O(s) H2O(l)are equal in magnitude and opposite in sign.
D)( G) is positive for the process H2O(s) H2O(l).
E)None of these is correct.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is true?
A)By spontaneous we mean that the reaction or process will always proceed to the right (as written)even if very slowly.Increasing the temperature may speed up the reaction,but it does not affect the spontaneity of the reaction.
B)By spontaneous we mean that the reaction or process will always proceed to the left (as written)even if very slowly.Increasing the temperature may speed up the reaction,but it does not affect the spontaneity of the reaction.
C)By spontaneous we mean that the reaction or process will always proceed to the left (as written)even if very slowly.Increasing the temperature may speed up the reaction and it generally affects the spontaneity of the reaction.
D)By spontaneous we mean that the reaction or process will always proceed to the right (as written)even if very slowly.Increasing the temperature may speed up the reaction,and it generally affects the spontaneity of the reaction.
E)None of the above is true.
A)By spontaneous we mean that the reaction or process will always proceed to the right (as written)even if very slowly.Increasing the temperature may speed up the reaction,but it does not affect the spontaneity of the reaction.
B)By spontaneous we mean that the reaction or process will always proceed to the left (as written)even if very slowly.Increasing the temperature may speed up the reaction,but it does not affect the spontaneity of the reaction.
C)By spontaneous we mean that the reaction or process will always proceed to the left (as written)even if very slowly.Increasing the temperature may speed up the reaction and it generally affects the spontaneity of the reaction.
D)By spontaneous we mean that the reaction or process will always proceed to the right (as written)even if very slowly.Increasing the temperature may speed up the reaction,and it generally affects the spontaneity of the reaction.
E)None of the above is true.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
Substance X has a heat of vaporization of 47.1 kJ/mol at its normal boiling point (423°C).For the process X(l) X(g)at 1 atm and 423°C calculate the value of S.
A)0
B)67.7 J/K mol
C)111 J/K mol
D)-67.7 J/K mol
E)-111 J/K mol
A)0
B)67.7 J/K mol
C)111 J/K mol
D)-67.7 J/K mol
E)-111 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is always true for a spontaneous process?
I. Ssys > 0
II. Ssurr > 0
III. Suniv > 0
IV. Gsys > 0
A)I
B)III
C)IV
D)I and III
E)III and IV
I. Ssys > 0
II. Ssurr > 0
III. Suniv > 0
IV. Gsys > 0
A)I
B)III
C)IV
D)I and III
E)III and IV

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
If Ssurr = - Ssys,the process is at equilibrium.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
As long as the disorder of the surroundings is increasing,a process will be spontaneous.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
For a spontaneous exothermic process,which of the following must be true?
A)( G) must be positive.
B)( S) must be positive.
C)( S) must be negative.
D)Two of the above must be true.
E)None of the above (A-C)must be true.
A)( G) must be positive.
B)( S) must be positive.
C)( S) must be negative.
D)Two of the above must be true.
E)None of the above (A-C)must be true.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
What must be true about G for this reaction?
A)( G = H
B)( G = 0)
C)( G > 0)
D)( G < 0)
E)( G) = Suniv
A)( G = H
B)( G = 0)
C)( G > 0)
D)( G < 0)
E)( G) = Suniv

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
As O2(l)is cooled at 1 atm,it freezes at 54.5 K to form Solid I.At a lower temperature,Solid I rearranges to Solid II,which has a different crystal structure.Thermal measurements show that H for the I II phase transition is -743.07 J/mol,and S for the same transition is -17.0 J/K mol.At what temperature are Solids I and II in equilibrium?
A)13.6 K
B)43.7 K
C)19.8 K
D)98.2 K
E)They can never be in equilibrium because they are both solids.
A)13.6 K
B)43.7 K
C)19.8 K
D)98.2 K
E)They can never be in equilibrium because they are both solids.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
When ignited,solid ammonium dichromate decomposes in a fiery display.This is the reaction for a "volcano" demonstration.The decomposition produces nitrogen gas,water vapor,and chromium(III)oxide.The temperature is constant at 25°C.
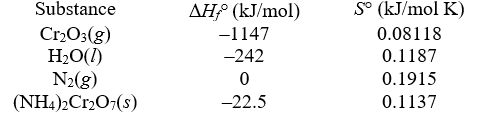
-Determine G° (in kJ/mol).
A)-191.4
B)-2281.4
C)-38.9
D)1903.6
E)-1555.4
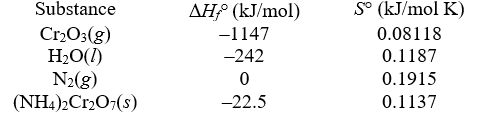
-Determine G° (in kJ/mol).
A)-191.4
B)-2281.4
C)-38.9
D)1903.6
E)-1555.4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
For a certain process at 355 K, G = -11.8 kJ and H = -9.2 kJ.Therefore, S for the process is
A)0 J/K mol
B)7.3 J/K mol
C)-7.3 J/K mol
D)-25.9 J/K mol
E)25.9 J/K mol
A)0 J/K mol
B)7.3 J/K mol
C)-7.3 J/K mol
D)-25.9 J/K mol
E)25.9 J/K mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
For a particular chemical reaction H = 4.3 kJ and S = -19 J/K.Under what temperature condition is the reaction spontaneous?
A)When T < -226 K.
B)When T < 226 K.
C)The reaction is spontaneous at all temperatures.
D)The reaction is not spontaneous at any temperature.
E)When T > 226 K.
A)When T < -226 K.
B)When T < 226 K.
C)The reaction is spontaneous at all temperatures.
D)The reaction is not spontaneous at any temperature.
E)When T > 226 K.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
The following reaction takes place at 120°C: H2O(l) H2O(g) H = 44.0 kJ/mol S = 0.119 kJ/mol K Which of the following must be true?
A)The reaction is not spontaneous.
B)The reaction is spontaneous.
C)( G = 0)
D)( G < 0)
E)Two of these.
A)The reaction is not spontaneous.
B)The reaction is spontaneous.
C)( G = 0)
D)( G < 0)
E)Two of these.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
A mixture of hydrogen and chlorine remains unreacted until it is exposed to ultraviolet light from a burning magnesium strip.Then the following reaction occurs very rapidly: H2(g)+ Cl2(g) 2HCl(g)
G = -45.54 kJ
H = -44.12 kJ
S = -4.76 J/K
Which of the following is consistent with this information?
A)The reactants are thermodynamically more stable than the products.
B)The reaction has a small equilibrium constant.
C)The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
D)The negative value for S slows down the reaction.
E)The reaction is spontaneous,but the reactants are kinetically stable.
G = -45.54 kJ
H = -44.12 kJ
S = -4.76 J/K
Which of the following is consistent with this information?
A)The reactants are thermodynamically more stable than the products.
B)The reaction has a small equilibrium constant.
C)The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
D)The negative value for S slows down the reaction.
E)The reaction is spontaneous,but the reactants are kinetically stable.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
The following questions refer to the following reaction at constant 25°C and 1 atm.
2Fe(s)+ (3/2)O2(g)+ 3H2O(l) 2Fe(OH)3(s) H = -789 kJ/mol

-Determine Ssurr for the reaction (in kJ/mol K)
A)3.14
B)0.937
C)0.378
D)1.31
E)2.65
2Fe(s)+ (3/2)O2(g)+ 3H2O(l) 2Fe(OH)3(s) H = -789 kJ/mol

-Determine Ssurr for the reaction (in kJ/mol K)
A)3.14
B)0.937
C)0.378
D)1.31
E)2.65

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction A + B C + D, H° = +40 kJ and S° = +50 J/K.Therefore,the reaction under standard conditions is
A)spontaneous at temperatures less than 10 K
B)spontaneous at temperatures greater than 800 K
C)spontaneous only at temperatures between 10 K and 800 K
D)spontaneous at all temperatures
E)nonspontaneous at all temperatures
A)spontaneous at temperatures less than 10 K
B)spontaneous at temperatures greater than 800 K
C)spontaneous only at temperatures between 10 K and 800 K
D)spontaneous at all temperatures
E)nonspontaneous at all temperatures

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
When ignited,solid ammonium dichromate decomposes in a fiery display.This is the reaction for a "volcano" demonstration.The decomposition produces nitrogen gas,water vapor,and chromium(III)oxide.The temperature is constant at 25°C.
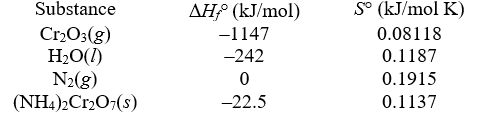
-Determine Suniv° (in kJ/mol K).
A)7.66
B)6.39
C)84.3
D)5.22
E)6.03
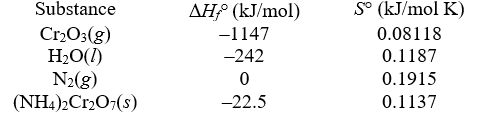
-Determine Suniv° (in kJ/mol K).
A)7.66
B)6.39
C)84.3
D)5.22
E)6.03

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the freezing of liquid water at -10°C.For this process what are the signs for H, S,and G?
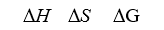
A)+ - 0
B)+ - -
C)- + 0
D)- + -
E)- - -
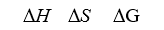
A)+ - 0
B)+ - -
C)- + 0
D)- + -
E)- - -

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
When ignited,solid ammonium dichromate decomposes in a fiery display.This is the reaction for a "volcano" demonstration.The decomposition produces nitrogen gas,water vapor,and chromium(III)oxide.The temperature is constant at 25°C.
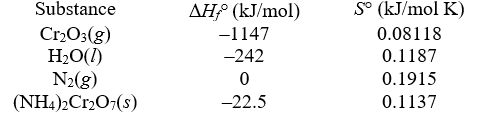
-Determine S° reaction (in kJ/mol K).
A)0.2777
B)0.8612
C)0.7475
D)0.6338
E)0.1590
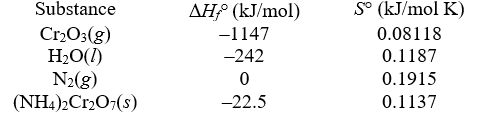
-Determine S° reaction (in kJ/mol K).
A)0.2777
B)0.8612
C)0.7475
D)0.6338
E)0.1590

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
At constant pressure,the following reaction 2NO2(g) N2O4(g)is exothermic.The reaction (as written)is
A)always spontaneous
B)spontaneous at low temperatures,but not high temperatures
C)spontaneous at high temperatures,but not low temperatures
D)never spontaneous
E)cannot tell
A)always spontaneous
B)spontaneous at low temperatures,but not high temperatures
C)spontaneous at high temperatures,but not low temperatures
D)never spontaneous
E)cannot tell

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
The third law of thermodynamics states:
A)The entropy of the universe is increasing.
B)The entropy of the universe is constant.
C)The entropy is zero at 0 K for a perfect crystal.
D)The absolute entropy of a substance decreases with increasing temperature.
E)The entropy of the universe equals the sum of the entropy of system and surroundings.
A)The entropy of the universe is increasing.
B)The entropy of the universe is constant.
C)The entropy is zero at 0 K for a perfect crystal.
D)The absolute entropy of a substance decreases with increasing temperature.
E)The entropy of the universe equals the sum of the entropy of system and surroundings.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
For the process of a certain liquid vaporizing at 1 atm, H°vap = 54.2 kJ/mol and S°vap= 74.1 J/mol K.Assuming these values are independent of T,what is the normal boiling point of this liquid?
A)731 °C
B)1004 °C
C)458 °C
D)0.731 °C
E)none of these
A)731 °C
B)1004 °C
C)458 °C
D)0.731 °C
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
In which case must a reaction be spontaneous at all temperatures?
A)( H) is positive, S is positive.
B)( H) = 0, S is negative.
C)( S) = 0, H is positive.
D)( H) is negative, S is positive.
E)None of these.
A)( H) is positive, S is positive.
B)( H) = 0, S is negative.
C)( S) = 0, H is positive.
D)( H) is negative, S is positive.
E)None of these.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
For the process S8 (rhombic) S8 (monoclinic)at 110°C, H = 3.21 kJ/mol and S = 8.70 J/K . mol (at 110°C). Which of the following is correct?
A)This reaction is spontaneous at 110°C (S8 (monoclinic)is stable).
B)This reaction is spontaneous at 110°C (S8 (rhombic)is stable).
C)This reaction is nonspontaneous at 110°C (S8 (rhombic)is stable).
D)This reaction is nonspontaneous at 110°C (S8 (monoclinic)is stable).
E)Need more data.
A)This reaction is spontaneous at 110°C (S8 (monoclinic)is stable).
B)This reaction is spontaneous at 110°C (S8 (rhombic)is stable).
C)This reaction is nonspontaneous at 110°C (S8 (rhombic)is stable).
D)This reaction is nonspontaneous at 110°C (S8 (monoclinic)is stable).
E)Need more data.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
The following questions refer to the following reaction at constant 25°C and 1 atm.
2Fe(s)+ (3/2)O2(g)+ 3H2O(l) 2Fe(OH)3(s) H = -789 kJ/mol

-Determine Suniv for the reaction (in kJ/mol K)
A)0.23
B)2.3
C)0.36
D)2.8
E)3.6
2Fe(s)+ (3/2)O2(g)+ 3H2O(l) 2Fe(OH)3(s) H = -789 kJ/mol

-Determine Suniv for the reaction (in kJ/mol K)
A)0.23
B)2.3
C)0.36
D)2.8
E)3.6

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
Given that Hvap is 53.3 kJ/mol,and the boiling point is 83.4°C,1 atm,if one mole of this substance is vaporized at 1 atm,calculate G.
A)-150 J
B)150 J
C)639 J
D)-639 J
E)0 J
A)-150 J
B)150 J
C)639 J
D)-639 J
E)0 J

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction is allowed to proceed until all substances involved have reached their equilibrium concentrations.Under those conditions,what is G for the reaction?
A)-1.35 105 kJ
B)98.7 kJ
C)-25.2 kJ
D)135 kJ
E)0
A)-1.35 105 kJ
B)98.7 kJ
C)-25.2 kJ
D)135 kJ
E)0

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
The standard molar free energies of formation of NO2(g)and N2O4(g)at 25°C are 51.84 and 98.00 kJ/mol,respectively.What is the value of G for the reaction written as follows at 25°C if the pressures of both gases are 1.33 atm? 2NO2  N2O4
N2O4
A)-4.97
B)4.97
C)-6.39
D)-5.68
E)-5.74
 N2O4
N2O4A)-4.97
B)4.97
C)-6.39
D)-5.68
E)-5.74

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
For the dissociation reaction of the acid HF: HF(aq)  H+(aq)+ F-(aq)
H+(aq)+ F-(aq)
S is observed to be negative.The best explanation is:
A)This is the expected result since each HF molecule produces two ions when it dissociates.
B)Hydration of the ions produces the negative value of S.
C)The reaction is expected to be exothermic and thus S should be negative.
D)The reaction is expected to be endothermic and thus S should be negative.
E)None of these can explain the negative value of S.
 H+(aq)+ F-(aq)
H+(aq)+ F-(aq)S is observed to be negative.The best explanation is:
A)This is the expected result since each HF molecule produces two ions when it dissociates.
B)Hydration of the ions produces the negative value of S.
C)The reaction is expected to be exothermic and thus S should be negative.
D)The reaction is expected to be endothermic and thus S should be negative.
E)None of these can explain the negative value of S.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is not a state function?
A)q
B)G
C)H
D)E
E)P
A)q
B)G
C)H
D)E
E)P

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the dissociation of hydrogen: H2(g)  2H(g)
2H(g)
One would expect that this reaction:
A)will be spontaneous at any temperature
B)will be spontaneous at high temperatures
C)will be spontaneous at low temperatures
D)will not be spontaneous at any temperature
E)will never happen
 2H(g)
2H(g)One would expect that this reaction:
A)will be spontaneous at any temperature
B)will be spontaneous at high temperatures
C)will be spontaneous at low temperatures
D)will not be spontaneous at any temperature
E)will never happen

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the reaction
2N2O5(g) 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)
at 25°C for which the following data are relevant:
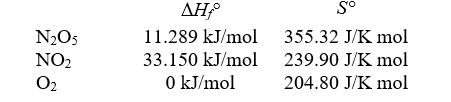
-Calculate G° for the reaction at 25°C.
A)-1.35 105 kJ
B)98.7 kJ
C)-25.2 kJ
D)135 kJ
E)0
2N2O5(g)
 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)at 25°C for which the following data are relevant:
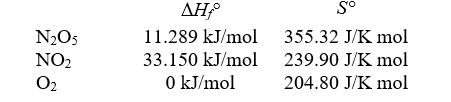
-Calculate G° for the reaction at 25°C.
A)-1.35 105 kJ
B)98.7 kJ
C)-25.2 kJ
D)135 kJ
E)0

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
In which reaction is S° expected to be positive?
A)I2(g) I2(s)
B)H2O(l) H2O(s)
C)CH3OH(g)+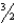 O2(g) CO2(g)+ 2H2O(l)
O2(g) CO2(g)+ 2H2O(l)
D)2O2(g)+ 2SO(g) 2SO3(g)
E)none of these
A)I2(g) I2(s)
B)H2O(l) H2O(s)
C)CH3OH(g)+
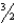 O2(g) CO2(g)+ 2H2O(l)
O2(g) CO2(g)+ 2H2O(l)D)2O2(g)+ 2SO(g) 2SO3(g)
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
For the reaction 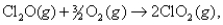 H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?
H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?
A)153.4 kJ/mol
B)47.6 kJ/mol
C)173.9 kJ/mol
D)78.9 kJ/mol
E)155.0 kJ/mol
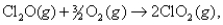 H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?
H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?A)153.4 kJ/mol
B)47.6 kJ/mol
C)173.9 kJ/mol
D)78.9 kJ/mol
E)155.0 kJ/mol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the reaction
2N2O5(g) 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)
at 25°C for which the following data are relevant:
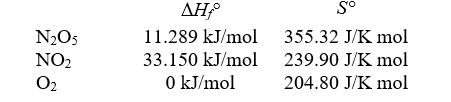
-Calculate S° for the reaction.
A)809.08 J/K
B)89.38 J/K
C)453.76 J/K
D)-265.94 J/K
E)1164.40 J/K
2N2O5(g)
 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)at 25°C for which the following data are relevant:
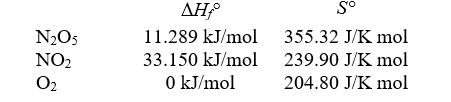
-Calculate S° for the reaction.
A)809.08 J/K
B)89.38 J/K
C)453.76 J/K
D)-265.94 J/K
E)1164.40 J/K

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
For which of the following processes would S° be expected to be most positive?
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(l) H2O(s)
C)NH3(g)+ HCl(g) NH4Cl(g)
D)2NH4NO3(s) 2N2(g)+ O2(g)+ 4H2O(g)
E)N2O4(g) 2NO2(g)
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(l) H2O(s)
C)NH3(g)+ HCl(g) NH4Cl(g)
D)2NH4NO3(s) 2N2(g)+ O2(g)+ 4H2O(g)
E)N2O4(g) 2NO2(g)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the reaction
2N2O5(g) 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)
at 25°C for which the following data are relevant:
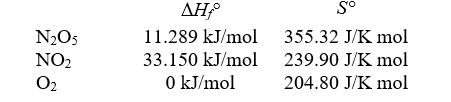
-Calculate H° for the reaction.
A)110.022 kJ
B)10.572 kJ
C)121.311 kJ
D)21.861 kJ
E)155.178 kJ
2N2O5(g)
 4NO2(g)+ O2(g)
4NO2(g)+ O2(g)at 25°C for which the following data are relevant:
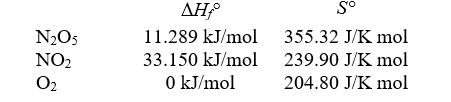
-Calculate H° for the reaction.
A)110.022 kJ
B)10.572 kJ
C)121.311 kJ
D)21.861 kJ
E)155.178 kJ

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
The standard free energy of formation of KCl(s)is -408.8 kJ/mol. G° for the reaction 2KCl(s) 2K(s)+ Cl2(g)is:
A)-408.8 kJ
B)817.6 kJ
C)408.8 kJ
D)-817.6 kJ
E)none of these
A)-408.8 kJ
B)817.6 kJ
C)408.8 kJ
D)-817.6 kJ
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the following hypothetical reaction at 310 K.Standard free energies of formation are given in parentheses. B C
G° = -32.6 kJ/mol(?)
(176.4 kJ/mol)
Calculate the standard free energy of formation of compound B.
A)209.0 kJ/mol
B)-209.0 kJ/mol
C)143.8 kJ/mol
D)-143.8 kJ/mol
E)none of these
G° = -32.6 kJ/mol(?)
(176.4 kJ/mol)
Calculate the standard free energy of formation of compound B.
A)209.0 kJ/mol
B)-209.0 kJ/mol
C)143.8 kJ/mol
D)-143.8 kJ/mol
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
Given the following data ( Hf,S°,respectively)for N2O4(l)-20.kJ/mol,209.0 J/K mol,and N2O4(g)10.kJ/mol,304.2 J/K mol.Above what temperature (in °C)is the vaporization of N2O4 liquid spontaneous?
A)Above -178 °C.
B)Above -231 °C.
C)Above 3 °C.
D)Above 30. °C.
E)Above 42 °C.
A)Above -178 °C.
B)Above -231 °C.
C)Above 3 °C.
D)Above 30. °C.
E)Above 42 °C.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
At 699 K, G° = -23.25 kJ for the reaction H2(g)+ I2(g)  2HI(g).Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.76 atm pressure.
2HI(g).Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.76 atm pressure.
A)-20.2 kJ
B)20.2 kJ
C)3.1 kJ
D)-43.4 kJ
E)43.4 kJ
 2HI(g).Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.76 atm pressure.
2HI(g).Calculate G for this reaction if the reagents are both supplied at 10.0 atm pressure and the product is at 1.76 atm pressure.A)-20.2 kJ
B)20.2 kJ
C)3.1 kJ
D)-43.4 kJ
E)43.4 kJ

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following statements is (are)always true?
I.In order for a process to be spontaneous,the entropy of the universe must increase.
II.A system cannot have both energy disorder and positional disorder.
III. Suniv =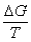 IV.S° is zero for elements in their standard states.
IV.S° is zero for elements in their standard states.
A)I
B)I,IV
C)I,III,IV
D)II,IV
E)II
I.In order for a process to be spontaneous,the entropy of the universe must increase.
II.A system cannot have both energy disorder and positional disorder.
III. Suniv =
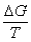 IV.S° is zero for elements in their standard states.
IV.S° is zero for elements in their standard states.A)I
B)I,IV
C)I,III,IV
D)II,IV
E)II

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
When a stable diatomic molecule spontaneously forms from its atoms,what are the signs of H°, S°,and G°?
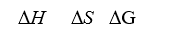
A)+ + +
B)+ - -
C)- + +
D)- - +
E)- - -
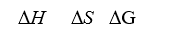
A)+ + +
B)+ - -
C)- + +
D)- - +
E)- - -

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is true for this reaction?
A)Both H° and S° favor the reaction's spontaneity.
B)Both H° and S° oppose the reaction's spontaneity.
C)( H°) favors the reaction,but S° opposes it.
D)( H°) opposes the reaction,but S° favors it.
E)The reaction cannot occur at room temperature.
A)Both H° and S° favor the reaction's spontaneity.
B)Both H° and S° oppose the reaction's spontaneity.
C)( H°) favors the reaction,but S° opposes it.
D)( H°) opposes the reaction,but S° favors it.
E)The reaction cannot occur at room temperature.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
In which process is S expected to be positive?
A)a reaction that forms a solid precipitant from aqueous solutions
B)an ideal gas being compressed at a constant temperature and against a constant pressure
C)water freezing below its normal freezing point
D)a spontaneous endothermic process at a constant temperature and pressure
E)none of these
A)a reaction that forms a solid precipitant from aqueous solutions
B)an ideal gas being compressed at a constant temperature and against a constant pressure
C)water freezing below its normal freezing point
D)a spontaneous endothermic process at a constant temperature and pressure
E)none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
Of S, Ssurr, Suniv,and G,which are state functions?
A)( S), Ssurr, Suniv,and G are all state functions.
B)Only S, Suniv,and G are state functions.
C)Only S and G are state functions.
D)Only S, Ssurr,and Suniv are state functions.
E)Only Suniv and G are state functions.
A)( S), Ssurr, Suniv,and G are all state functions.
B)Only S, Suniv,and G are state functions.
C)Only S and G are state functions.
D)Only S, Ssurr,and Suniv are state functions.
E)Only Suniv and G are state functions.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck


