Deck 12: The Mucosal Immune System
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 12: The Mucosal Immune System
1
NOD proteins are intracellular sensors of infections that recognize cell wall or peptidoglycan components of many bacteria. The importance of these sensors in maintaining homeostasis in the intestine is indicated by the consequences for individuals lacking one of these sensors, NOD2. Specifically, NOD2 mutations have been found in a large percentage of individuals with a form of inflammatory bowel disease known as Crohn's disease. How might the absence of NOD2 lead to loss of barrier integrity and thus to increased inflammation in the gastrointestinal epithelium?
NOD2 binds to peptidoglycan that is released from the gut microbiota and then signals to activate the transcription factor NF B. NF B activation leads to production of inflammatory cytokines and chemokines, antimicrobial peptides and mucin. This is considered a healthy, steady-state level of inflammation, that serves to restrain the microbiota, create physical separation between the microbes and the gut epithelial cells, and leads to enhanced barrier function. In Crohn's disease that is associated with NOD2 mutations, this process breaks down and the intestinal barrier is compromised. While the details of these changes are not fully known, the outcome of chronic inflammation likely reflects increased stimulation of the immune cells in the gut epithelium by over-exposure to commensal or pathogenic microbes.
2
Salmonella typhimurium is the causative agent of typhoid fever, and infects the host by translocating across the intestinal epithelium. Recent studies have shown that S. typhimurium produces an effector protein called SopB that induces intestinal enterocytes to differentiate into M cells. This is beneficial to the bacteria because:
A) S. typhimurium gains access to the host by crossing the intestinal epithelium inside M cells.
B) M cells are unable to secrete antimicrobial peptides as do the enterocytes.
C) M cells are unable to secrete mucus as do the enterocytes.
D) S. typhimurium utilizes the M cell metabolic machinery for its proliferation.
E) M cells lack the pattern recognition receptors that induce innate responses to pathogens.
A) S. typhimurium gains access to the host by crossing the intestinal epithelium inside M cells.
B) M cells are unable to secrete antimicrobial peptides as do the enterocytes.
C) M cells are unable to secrete mucus as do the enterocytes.
D) S. typhimurium utilizes the M cell metabolic machinery for its proliferation.
E) M cells lack the pattern recognition receptors that induce innate responses to pathogens.
S. typhimurium gains access to the host by crossing the intestinal epithelium inside M cells.
3
Purified CD4 T cells were stimulated in vitro with anti-CD3 plus anti-CD28 antibodies to activate them, and cultured for two days in the presence or absence of all-trans retinoic acid (RA). The T cells were then stained with antibodies to either a 4: 7 integrin or for E/P-selectin ligand expression and analyzed by flow cytometry. The results are shown in the upper graph in Figure. In a second assay, the cells were tested for their ability to migrate in response to a panel of chemokines, and the percentages of migrated cells were measured, as shown in the lower graph. Note that these chemokines are ligands for the receptors as indicated in Figure. In the absence of infection or inflammation, where are naive T cells encountering RA, and based on these data, what effect will this have on their subsequent behavior? 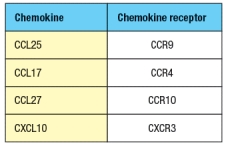
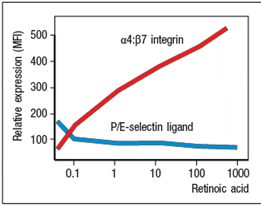
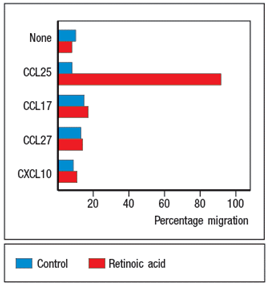



In the absence of infection or inflammation, the encounter between migrating dendritic cells and naive T cells in mesenteric lymph nodes is the generation of antigen-specific FoxP3+ regulatory T cells that express the gut-homing molecules CCR9 and integrin 4: 7. These 'primed' Treg cells then leave the lymph node and return to the wall of the small intestine.
The expression of gut-homing molecules require that the dendritic cells produce retinoic acid, which is derived from the metabolism of dietary vitamin A through the action of retinal dehydrogenases. Retinoic acid is also produced by stromal cells in the mesenteric lymph node, further enhancing the effects of the migratory dendritic cells. Retinoic acid-producing dendritic cells are also found in Peyer's patches.
The expression of gut-homing molecules require that the dendritic cells produce retinoic acid, which is derived from the metabolism of dietary vitamin A through the action of retinal dehydrogenases. Retinoic acid is also produced by stromal cells in the mesenteric lymph node, further enhancing the effects of the migratory dendritic cells. Retinoic acid-producing dendritic cells are also found in Peyer's patches.
4
Analysis of human milk from lactating mothers shows that it contains IgA antibodies against infections that were recent (<3 weeks earlier) and those from the distant past (>1 year). These antibodies are directed against a host of organisms, including viruses, such as enteroviruses, herpes simplex viruses, respiratory syncytial virus, rubella, reovirus, and rotavirus. In addition, IgA antibodies against many bacteria are found in human milk, including those reactive to E. coli, Shigella, Salmonella, Campylobacter, Vibrio cholerae, H. influenzae, S. pneumoniae, Clostridium difficile, C. botulinum, and Klebsiella pneumoniae. IgA antibodies to the parasite Giardia and the fungus, Candida albicans, are also seen in human milk. Since most of these infections were localized in the gastrointestinal tract of the mother, these IgA antibodies ended up in breast milk by:
A) Being transported from the lymph fluid in the breast tissue into across the breast epithelium into the secretory glands.
B) The trafficking of germinal center B cells from the mother's mesenteric lymph nodes to the breast epithelium.
C) The trafficking of gut-primed activated B cells from the mother's circulation into the lactating milk gland.
D) The ability of gut-primed activated B cells to traffic to all secondary lymphoid tissues in the mother.
E) The ability of activated B cells primed in the spleen to switch to IgA secretion after entering the mother's lactating milk gland.
A) Being transported from the lymph fluid in the breast tissue into across the breast epithelium into the secretory glands.
B) The trafficking of germinal center B cells from the mother's mesenteric lymph nodes to the breast epithelium.
C) The trafficking of gut-primed activated B cells from the mother's circulation into the lactating milk gland.
D) The ability of gut-primed activated B cells to traffic to all secondary lymphoid tissues in the mother.
E) The ability of activated B cells primed in the spleen to switch to IgA secretion after entering the mother's lactating milk gland.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
Mice lacking the poly Ig receptor (pIgR) have an immunodeficiency disease characterized by increased susceptibility to mucosal infections and an increase in the penetration of commensal microbes into the body's tissues. Yet, a genetic deficiency in the production of IgA antibodies is the most common form of human immunodeficiency, and is generally a mild disease and often even asymptomatic. This dichotomy can be explained by:
A) The difference in the immune system between mice and humans
B) The different types of commensal microbes found in mice and humans
C) The ability of pIgR to transport IgM across the gut epithelium
D) The reduced exposure of humans compared to mice to pathogens that infect via the gastrointestinal epithelium
E) The development of improved human hygiene, including pasteurization
A) The difference in the immune system between mice and humans
B) The different types of commensal microbes found in mice and humans
C) The ability of pIgR to transport IgM across the gut epithelium
D) The reduced exposure of humans compared to mice to pathogens that infect via the gastrointestinal epithelium
E) The development of improved human hygiene, including pasteurization

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
Infection of mice with the bacterial pathogen, Citrobacter rodentium, elicits a protective immune response in the gastrointestinal tract. This protective response is characterized by two sequential waves of IL-22 production, both induced by IL-23. The early wave of IL-22 production (
A) Lamina propria dendritic cells responding to PAMPs of the bacterial pathogen
B) Lamina propria macrophages responding to PAMPs of the bacterial pathogen
C) Naive CD4 T cells induced to differentiate into TH17 cells in the mesenteric lymph node
D) Lamina propria ILC3 cells stimulated by dendritic cell-produced IL-23
E) IgA immune complexes transporting bacterial antigens and stimulating lamina propria dendritic cells to produce IL-22
A) Lamina propria dendritic cells responding to PAMPs of the bacterial pathogen
B) Lamina propria macrophages responding to PAMPs of the bacterial pathogen
C) Naive CD4 T cells induced to differentiate into TH17 cells in the mesenteric lymph node
D) Lamina propria ILC3 cells stimulated by dendritic cell-produced IL-23
E) IgA immune complexes transporting bacterial antigens and stimulating lamina propria dendritic cells to produce IL-22

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
Oral inoculation with rotavirus, an intestinal pathogen, induces adaptive immune responses that are initiated in gut-associated lymphoid tissue, such as mesenteric lymph nodes and Peyer's patches. The rotavirus-specific effector T cells that are generated in this response are never found in the circulation, but home directly from the lymphoid tissue to the epithelium without ever leaving the intestinal environment.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
The TACI receptor on B cells, which binds to BAFF and APRIL, is important in IgA antibody secretion by B cells in the gastrointestinal lamina propria.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
Patients receiving hematopoietic stem cell transplants often suffer from graft-versus-host disease (GVHD), involving immune-mediated damage to the gastrointestinal (GI) tract. The symptoms of GVHD include nausea, vomiting, diarrhea, and abdominal cramping due to epithelial cell apoptosis and inflammatory leukocyte infiltration into the GI epithelium. One proposed treatment has been tested in mouse models of GVHD for potential therapeutic benefit. This treatment is:
A) Administration of IL-22
B) Administration of anti-microbial peptides
C) Administration of mucosal (M2) macrophages
D) Administration of TGF-
E) Administration of IL-1 + IL-6
A) Administration of IL-22
B) Administration of anti-microbial peptides
C) Administration of mucosal (M2) macrophages
D) Administration of TGF-
E) Administration of IL-1 + IL-6

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
The mucosal immune system provides protection for a vast area of the body and contains three-quarters of all the lymphocytes in the body. A key feature of the mucosal immune system is its:
A) Ability to respond to thousands of different bacterial species
B) Inability to provide adequate protection, leading to millions of deaths per year due to mucosal infections
C) Ability to avoid responding to the large numbers and differing species of commensal microbes
D) Use of the identical mechanisms and response features compared to the systemic immune system
E) Inability to produce antibodies that cross epithelial barriers
A) Ability to respond to thousands of different bacterial species
B) Inability to provide adequate protection, leading to millions of deaths per year due to mucosal infections
C) Ability to avoid responding to the large numbers and differing species of commensal microbes
D) Use of the identical mechanisms and response features compared to the systemic immune system
E) Inability to produce antibodies that cross epithelial barriers

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
Vibrio cholerae causes an acute diarrheal illness that can be fatal if not treated. Several vaccines have been developed in an effort to prevent this disease. The oral cholera vaccine is a mixture of killed Vibrio cholerae bacteria plus additional inactivated cholera toxin protein. Efficacy studies of this vaccine indicate that it prevents 50-60% of the cases of cholera infection observed in non-vaccinated individuals. In contrast, injectable vaccines made from killed bacteria or purified bacterial subunits are substantially less effective at preventing infections. This is likely due to the fact that:
A) The injectable vaccine fails to elicit gut-homing immune responses.
B) The oral vaccine lasts substantially longer in the body than the injectable vaccine.
C) The oral vaccine has a higher concentration of bacterial antigens than the injectable vaccine.
D) The oral vaccine contains the inactivated cholera toxin.
E) The injectable vaccine does not contain protein epitopes to elicit CD4 helper T cells.
A) The injectable vaccine fails to elicit gut-homing immune responses.
B) The oral vaccine lasts substantially longer in the body than the injectable vaccine.
C) The oral vaccine has a higher concentration of bacterial antigens than the injectable vaccine.
D) The oral vaccine contains the inactivated cholera toxin.
E) The injectable vaccine does not contain protein epitopes to elicit CD4 helper T cells.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
A healthy intestinal mucosa is one in which induced adaptive immune responses to pathogenic infections are balanced by the lack of responses to innocuous food antigens and commensal microbes. This balance is maintained by an array of different subsets of effector T cells and regulatory T cells that reside in the intestinal epithelium and lamina propria. Although these different T cell subsets have diverse patterns of cytokine production and other effector functions, they share:
A) The ability to live for months to years in the intestinal epithelium
B) The ability to secrete immunosuppressive cytokines
C) The ability to inactivate dendritic cells that have received signals through pattern recognition receptors
D) The expression of gut-homing chemokine receptor, CCR9
E) The ability to bind to E-cadherin on the intestinal epithelial cells
A) The ability to live for months to years in the intestinal epithelium
B) The ability to secrete immunosuppressive cytokines
C) The ability to inactivate dendritic cells that have received signals through pattern recognition receptors
D) The expression of gut-homing chemokine receptor, CCR9
E) The ability to bind to E-cadherin on the intestinal epithelial cells

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
Mice deficient in the enzyme MMP7, that cleaves prepro- -defensin to the active -defensin, show an increased susceptibility to the enteric pathogen, Salmonella typhimurium. The cell type in the gastrointestinal (GI) epithelium most likely to express the highest levels of MMP7 is:
A) Goblet cells
B) Paneth cells
C) M cells
D) GI-resident macrophages
E) Intraepithelial lymphocytes
A) Goblet cells
B) Paneth cells
C) M cells
D) GI-resident macrophages
E) Intraepithelial lymphocytes

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
Mice lacking IL-15 or the IL-15R chain have substantially reduced numbers of type b IELs, including both : and : T-cell receptor-positive subsets. Normal numbers of type b IELs can be restored in il15-/- mice by cell-type specific expression of IL-15 in:
A) Thymic medullary epithelial cells
B) Thymic cortical epithelial cells
C) Dendritic cells
D) CD8 + T cells
E) Intestinal epithelial cells
A) Thymic medullary epithelial cells
B) Thymic cortical epithelial cells
C) Dendritic cells
D) CD8 + T cells
E) Intestinal epithelial cells

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
The Fc receptor FcRn, expressed in intestinal epithelial cells, binds to the Fc portion of IgG antibodies. Moreover, FcRn binds to IgG with high affinity at an acidic pH (<6.5) but not at physiological pH (pH 7.4). How is this biochemical feature of FcRn critical for its role in antigen presentations for gut immunity?

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
In addition to Peyer's patches that resemble systemic lymph nodes, the intestinal epithelium also contains several thousand isolated lymphoid follicles. Unlike the organized secondary lymphoid tissues in the mucosal immune system, these lymphoid follicles:
A) Contain mostly T cells and very few B cells
B) Develop only after birth in response to colonization by commensal microbes
C) Are not connected to the lymphatic system that drains to lymph nodes
D) Have no M cells to deliver gut antigens to the lymphocytes in the follicles
E) Are found only in the intestinal epithelium, and not in other mucosal epithelia
A) Contain mostly T cells and very few B cells
B) Develop only after birth in response to colonization by commensal microbes
C) Are not connected to the lymphatic system that drains to lymph nodes
D) Have no M cells to deliver gut antigens to the lymphocytes in the follicles
E) Are found only in the intestinal epithelium, and not in other mucosal epithelia

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
Reovirus is an enteric virus that infects mice by adhering to intestinal M cells and then using M cell transport to enter Peyer's patches. Mice that were orally inoculated with reovirus cleared the primary infection, and upon secondary challenge 21 days later, had no detectable virus in their Peyer's patches. In contrast, naive controls that did not receive the primary inoculation had >103 PFU of virus/mg of tissue following oral challenge with virus. The protective response induced by the primary oral inoculation would also be eliminated in:
A) FcRn-deficient mice
B) IgA-deficient mice
C) IgM-deficient mice
D) Fc RI-deficient mice
E) IgG-deficient mice
A) FcRn-deficient mice
B) IgA-deficient mice
C) IgM-deficient mice
D) Fc RI-deficient mice
E) IgG-deficient mice

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
In a second experiment, the isolated splenic and lamina propria macrophages were treated with LPS and then incubated with the protein antigen, chicken ovalbumin. CD4 T cells specific for a peptide of ovalbumin (OVA) bound to MHC class II were then incubated with each population of macrophages, and three days later, the culture supernatants were analyzed for IL-2 and IFN- production. Which of the graphs in Figure most likely represents the results of this experiment? 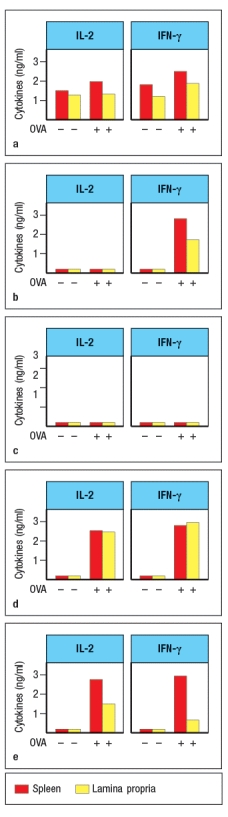


Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
Macrophages were isolated from the spleen or the intestinal lamina propria, and stimulated in vitro with the indicated TLR ligands. Twenty-four hours later, the culture supernatants were tested for the amounts of IL-10, the IL-12p40 subunit (shared between IL-12 and IL-23), and for IL-12, and the results are shown in Figure . 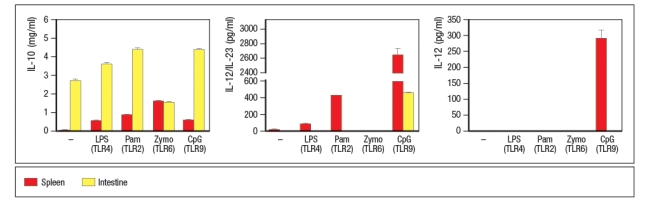
These data indicate that the lamina propria macrophages:
A) Make robust responses to a variety of MAMPs
B) Function as anti-inflammatory cells
C) Are unable to respond to TLR stimulation
D) Are less efficient at cytokine production than splenic macrophages
E) Function to promote epithelial cell repair in case of disruptions in the intestinal barrier

These data indicate that the lamina propria macrophages:
A) Make robust responses to a variety of MAMPs
B) Function as anti-inflammatory cells
C) Are unable to respond to TLR stimulation
D) Are less efficient at cytokine production than splenic macrophages
E) Function to promote epithelial cell repair in case of disruptions in the intestinal barrier

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
Secretory IgA produced in the epithelium of mucosal surfaces has several functions in protective immunity. Among these are neutralization of pathogens or toxins in the gastrointestinal tract lumen or in epithelial cell endosomes, neutralization of pathogens or toxins that cross the epithelial barrier, and transport of pathogens or toxins across M cells for delivery to lamina propria dendritic cells. All of these functions share the common feature that they:
A) Fail to induce local inflammation in the gastrointestinal epithelium
B) Are efficient at inducing opsonization of pathogens for uptake by phagocytes
C) Are efficient at inducing complement activation
D) Fail to completely protect the epithelium from cytotoxic effects of pathogens
E) Induce IgA-secreting plasma cells to produce increased amounts of secretory IgA
A) Fail to induce local inflammation in the gastrointestinal epithelium
B) Are efficient at inducing opsonization of pathogens for uptake by phagocytes
C) Are efficient at inducing complement activation
D) Fail to completely protect the epithelium from cytotoxic effects of pathogens
E) Induce IgA-secreting plasma cells to produce increased amounts of secretory IgA

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
Recurrent infections with the enteric pathogen Clostridium difficile is a major cause of chronic antibiotic-associated diarrhea, and causes >10,000 deaths per year in the US. An increasing number of individuals with this disorder are choosing to be treated with a fecal transplant from a healthy individual. Why is this treatment effective?

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
Wild-type mice are adoptively transferred with naive CD4 T cells isolated from OT-II T-cell receptor transgenic mice. These OT-II T cells are specific for a peptide of the chicken ovalbumin protein (OVA) bound to MHC class II. After transfer, the OT-II cells rapidly disperse to all of the secondary lymphoid organs in the recipient mice. Half of the recipient mice are then given OVA protein dissolved in their drinking water, and the other half of the mice receive normal drinking water for 5 days. On the following day (day 6), lymphocytes from the spleen, the peripheral lymph nodes (LN), mesenteric lymph nodes (MLN), Peyer's patches (PP) and lamina propria (LP) are isolated and examined for the proportions of CD4 T cells in each organ expressing FoxP3. The results are shown in Figure. 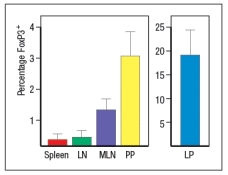
a) What is the conclusion from this experiment?
To investigate this further, dendritic cells are isolated from the spleen (spl-DC) or from the lamina propria of the small intestine (LP-DC). Each subset of dendritic cells is pulsed with the OVA protein, and then incubated with naive OT-II CD4 T cells for 5 days in the presence or absence of TGF- . The CD4 T cells are then examined for FoxP3 expression; the results are shown in Figure.
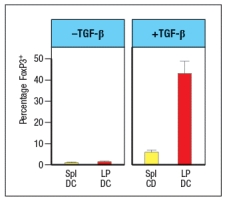
b) Why is TGF- added in this experiment?
c) What is the major difference between the splenic dendritic cells and those isolated from the lamina propria?
The CD4 T cells from the experiment above, in which the T cells were cultured for 5 days with OVA-pulsed splenic or lamina propria dendritic cells in the presence or absence of TGF- , were also assessed for their surface expression of the integrin 4 7.
d) Of the four groups of T cells shown in the graph, which ones would be expected to show high levels of 4 7?

a) What is the conclusion from this experiment?
To investigate this further, dendritic cells are isolated from the spleen (spl-DC) or from the lamina propria of the small intestine (LP-DC). Each subset of dendritic cells is pulsed with the OVA protein, and then incubated with naive OT-II CD4 T cells for 5 days in the presence or absence of TGF- . The CD4 T cells are then examined for FoxP3 expression; the results are shown in Figure.

b) Why is TGF- added in this experiment?
c) What is the major difference between the splenic dendritic cells and those isolated from the lamina propria?
The CD4 T cells from the experiment above, in which the T cells were cultured for 5 days with OVA-pulsed splenic or lamina propria dendritic cells in the presence or absence of TGF- , were also assessed for their surface expression of the integrin 4 7.
d) Of the four groups of T cells shown in the graph, which ones would be expected to show high levels of 4 7?

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
Oral tolerance to food antigens and immune tolerance to gut microbiota share the property that foreign antigens encountered in the gastrointestinal (GI) tract-food and commensal microbes, respectively-do not elicit immune effector responses. Yet, these processes differ in that commensal microbes will still elicit protective adaptive immune responses if they cross the GI epithelium and enter the body.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
Like wild-type (WT) mice, Rag-deficient mice have large numbers of commensal microbes in their gastrointestinal (GI) tract that remain contained in restricted to anatomical locations associated with the GI tract. However, when Rag-deficient mice are treated with a depleting antibody that eliminates all lymphocytes from the animals, these mice suffer from dissemination of commensal bacteria to peripheral organs, such as spleen and liver, and show evidence of systemic inflammation as shown by cytokine levels in the serum. ND, not detected; D3, day 3 post-antibody injection; D14, day 14 post-antibody injection. 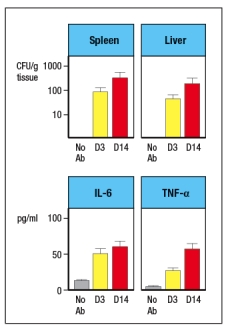
a) What are the cells in Rag-deficient mice, that, when depleted by the antibody injection, cause bacterial dissemination and systemic inflammation?
In a follow-up study, the Rag-deficient mice were treated with a blocking antibody to neutralize a cytokine (cytokine Z) known to be produced by lymphocytes. On day 14 post-antibody injection, the spleen and liver of the mice were analyzed for numbers of bacteria, and the results are shown in Figure :
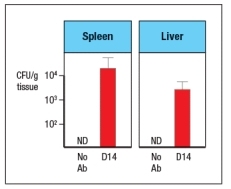
b) What is the likely identity of cytokine Z?
In a second follow-up study, Rag-deficient mice were crossed to a knockout for a transcription factor (Factor X) also known to be expressed in some lymphocytes. Lamina propria lymphocytes were isolated from wild-type mice, Rag-deficient mice, Factor-X-deficient mice, and double Rag/Factor-X deficient mice, and mRNA was prepared from the cells. RT-PCR was then used to determine the mRNA transcript levels for cytokine Z in all four lymphocyte populations, and the results are shown in Figure.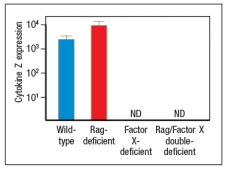
c) What is the likely identity of Factor X?
In a final experiment, the entire small intestine of wild-type mice, Rag-deficient mice, or Factor X-deficient mice is isolated and processed for mRNA, which is then used in RT-PCR experiments. Expression levels of mRNA encoding RegIII and RegIII are assessed in these samples; the results are shown in Figure.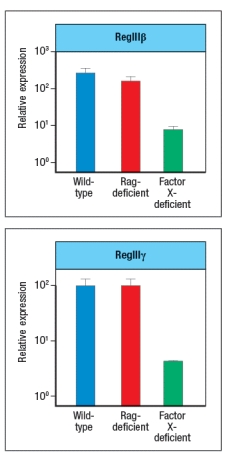
d) What do these data indicate about the regulation of RegIII and RegIII expression? Which cells are expressing RegIII and RegIII ?

a) What are the cells in Rag-deficient mice, that, when depleted by the antibody injection, cause bacterial dissemination and systemic inflammation?
In a follow-up study, the Rag-deficient mice were treated with a blocking antibody to neutralize a cytokine (cytokine Z) known to be produced by lymphocytes. On day 14 post-antibody injection, the spleen and liver of the mice were analyzed for numbers of bacteria, and the results are shown in Figure :

b) What is the likely identity of cytokine Z?
In a second follow-up study, Rag-deficient mice were crossed to a knockout for a transcription factor (Factor X) also known to be expressed in some lymphocytes. Lamina propria lymphocytes were isolated from wild-type mice, Rag-deficient mice, Factor-X-deficient mice, and double Rag/Factor-X deficient mice, and mRNA was prepared from the cells. RT-PCR was then used to determine the mRNA transcript levels for cytokine Z in all four lymphocyte populations, and the results are shown in Figure.

c) What is the likely identity of Factor X?
In a final experiment, the entire small intestine of wild-type mice, Rag-deficient mice, or Factor X-deficient mice is isolated and processed for mRNA, which is then used in RT-PCR experiments. Expression levels of mRNA encoding RegIII and RegIII are assessed in these samples; the results are shown in Figure.

d) What do these data indicate about the regulation of RegIII and RegIII expression? Which cells are expressing RegIII and RegIII ?

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
IL-10-deficient mice develop spontaneous colitis, a disease due to chronic inflammation in the gastrointestinal (GI) tract. However, when these mice are housed in germ-free conditions, in which they lack all commensal microbes in their GI tract, no colitis was observed. In addition, one would expect germ-free IL-10-deficient mice to also show:
A) Evidence of enhanced systemic immune activation compared to conventionally housed il10-/- mice
B) Enlarged mesenteric lymph nodes compared to conventionally housed il10-/- mice
C) Elevated numbers of FoxP3+ T cells in the lamina propria compared to conventionally housed il10-/- mice
D) Increased production of IgG antibodies compared to conventionally housed il10-/- mice
E) Reduced production of IgA antibodies compared to conventionally housed il10-/- mice
A) Evidence of enhanced systemic immune activation compared to conventionally housed il10-/- mice
B) Enlarged mesenteric lymph nodes compared to conventionally housed il10-/- mice
C) Elevated numbers of FoxP3+ T cells in the lamina propria compared to conventionally housed il10-/- mice
D) Increased production of IgG antibodies compared to conventionally housed il10-/- mice
E) Reduced production of IgA antibodies compared to conventionally housed il10-/- mice

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
When mice are born, their intestinal lamina propria lacks TH17 effector cells. These cells develop after birth due to:
A) Stimulation by antigens derived from commensal microbes that populate the gastrointestinal tract after birth
B) Stimulation by retinoic acid from dietary sources
C) Stimulation by IL-10 produced from FoxP3+ regulatory T cells
D) Stimulation by short-chain fatty acids derived from commensal microbes that populate the gastrointestinal tract after birth
E) Stimulation by antimicrobial peptides produced by intestinal epithelial cells
A) Stimulation by antigens derived from commensal microbes that populate the gastrointestinal tract after birth
B) Stimulation by retinoic acid from dietary sources
C) Stimulation by IL-10 produced from FoxP3+ regulatory T cells
D) Stimulation by short-chain fatty acids derived from commensal microbes that populate the gastrointestinal tract after birth
E) Stimulation by antimicrobial peptides produced by intestinal epithelial cells

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
IPEX syndrome is a genetic disease due to mutations that disrupt the function of an important transcription factor expressed in subset of CD4 T cells. Individuals with this disease generally begin showing symptoms shortly after birth. This disease is often fatal. One of the most prominent symptoms of IPEX is severe gastrointestinal inflammation accompanied by severe diarrhea. This transcription factor is most likely:
A) ROR t
B) T-bet
C) STAT3
D) NF B
E) FoxP3
A) ROR t
B) T-bet
C) STAT3
D) NF B
E) FoxP3

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


