Deck 2: The Chemical Context of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/137
Play
Full screen (f)
Deck 2: The Chemical Context of Life
1
Two atoms that have the same mass number must have the same
A) atomic number.
B) number of electrons.
C) protons.
D) number of protons + neutrons.
E) chemical properties.
A) atomic number.
B) number of electrons.
C) protons.
D) number of protons + neutrons.
E) chemical properties.
D
2
In what way are elements in the same row of the periodic table the same?
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.
E
3
The atomic number of sulfur is 16, which indicates that a sulfur atom contains
A) 16 neutrons.
B) 16 protons.
C) 16 protons and 16 neutrons.
D) 8 electrons in its outermost electron shell.
E) 8 protons and 8 neutrons.
A) 16 neutrons.
B) 16 protons.
C) 16 protons and 16 neutrons.
D) 8 electrons in its outermost electron shell.
E) 8 protons and 8 neutrons.
B
4
Argon has atomic number 18. Which of the following statements about argon is true?
A) It has 10 electrons in its outer electron shell.
B) It is inert.
C) It has an atomic mass of 18 daltons.
D) It has six valence electrons.
E) It resides in the first column of the periodic table.
A) It has 10 electrons in its outer electron shell.
B) It is inert.
C) It has an atomic mass of 18 daltons.
D) It has six valence electrons.
E) It resides in the first column of the periodic table.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
5
In what way are elements in the same column of the periodic table the same?
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.
A) They have the same number of protons.
B) They have the same number of neutrons.
C) They have the same number of electrons.
D) They have the same number of electrons in their valence shell.
E) They have the same number of electron shells.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
6
About 20-25% of the 92 natural elements are known to be essential to life. Which four of these elements make up approximately 96% of living matter?
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium
A) carbon, sodium, hydrogen, nitrogen
B) carbon, oxygen, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, nitrogen, calcium

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
7
For elements in the first three rows of the periodic table, the position of an element in the row indicates which of the following?
A) the total number of electrons in the element
B) the total number of protons in the element
C) the total number of neutrons in the element
D) the number of electron orbitals in the element
E) the number of electrons in the valence shell
A) the total number of electrons in the element
B) the total number of protons in the element
C) the total number of neutrons in the element
D) the number of electron orbitals in the element
E) the number of electrons in the valence shell

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
8
Oxygen has an atomic number of 8 and a mass number of 16. What is the atomic mass of an oxygen atom?
A) approximately 8 grams
B) approximately 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) approximately 24 grams
A) approximately 8 grams
B) approximately 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) approximately 24 grams

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
9
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by humans and other vertebrates?
A) nitrogen
B) calcium
C) iodine
D) sodium
E) phosphorus
A) nitrogen
B) calcium
C) iodine
D) sodium
E) phosphorus

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
10
From the atomic mass, one can deduce the number of ________ in each atom of an element.
A) protons
B) neutrons
C) electrons
D) protons plus electrons
E) protons plus neutrons
A) protons
B) neutrons
C) electrons
D) protons plus electrons
E) protons plus neutrons

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
11
An atom has 6 electrons in its outer shell. How many unpaired electrons does it have?
A) 0
B) 2
C) 4
D) 6
E) 2 or 4
A) 0
B) 2
C) 4
D) 6
E) 2 or 4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
12
Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers of 92, 94, 95, 96, 97, 98, and 100. Therefore, which of the following is true?
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different electron configurations.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum have between 50 and 58 neutrons and have different electron configurations.
E) The isotopes of molybdenum have between 50 and 58 protons and have different electron configurations.
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) The isotopes of molybdenum have different electron configurations.
C) The isotopes of molybdenum can have between 50 and 58 protons.
D) The isotopes of molybdenum have between 50 and 58 neutrons and have different electron configurations.
E) The isotopes of molybdenum have between 50 and 58 protons and have different electron configurations.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is primarily responsible for the unique chemical properties of each element?
A) Each element has a unique atomic mass.
B) Each element has a unique atomic number.
C) Each element has a unique number of protons.
D) Each element has a unique number of neutrons.
E) Each element has unique radioactive properties.
A) Each element has a unique atomic mass.
B) Each element has a unique atomic number.
C) Each element has a unique number of protons.
D) Each element has a unique number of neutrons.
E) Each element has unique radioactive properties.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
14
The chemical behavior of an atom depends primarily upon which of the following?
A) the number of neutrons in the nucleus
B) the number of protons in the nucleus
C) the number of electrons in the valence shell
D) the total number of electrons contained by the atom
E) the number of electron shells contained by the atom
A) the number of neutrons in the nucleus
B) the number of protons in the nucleus
C) the number of electrons in the valence shell
D) the total number of electrons contained by the atom
E) the number of electron shells contained by the atom

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
15
Phosphorus-32, a radioactive isotope of phosphorus-31 (atomic number 15), undergoes a form of radioactive decay whereby a neutron turns into a proton, which is retained in the nucleus, and emits radiation in the form of an electron. What is the product of such radioactive decay of phosphorus-32?
A) phosphorus-31
B) a positively charged phosphorus-31 ion
C) a negatively charged phosphorus-32 ion
D) sulfur-32 (atomic number 16)
E) the conversion of the phosphorus-32 atom into pure energy
A) phosphorus-31
B) a positively charged phosphorus-31 ion
C) a negatively charged phosphorus-32 ion
D) sulfur-32 (atomic number 16)
E) the conversion of the phosphorus-32 atom into pure energy

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
16
The nucleus of a nitrogen atom contains 7 neutrons and 7 protons. Which of the following is a correct statement concerning nitrogen?
A) The nitrogen atom has a mass number of 7 and an atomic number of 14.
B) The nitrogen atom has a mass number of 14 and an atomic number of 7.
C) The nitrogen atom has a mass number of 14 and an atomic number of 14.
D) The nitrogen atom has a mass number of 7 and an atomic number of 21.
E) The nitrogen atom has a mass number of 14 and an atomic number of 21.
A) The nitrogen atom has a mass number of 7 and an atomic number of 14.
B) The nitrogen atom has a mass number of 14 and an atomic number of 7.
C) The nitrogen atom has a mass number of 14 and an atomic number of 14.
D) The nitrogen atom has a mass number of 7 and an atomic number of 21.
E) The nitrogen atom has a mass number of 14 and an atomic number of 21.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
17
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by all organisms?
A) iron
B) calcium
C) iodine
D) sodium
E) potassium
A) iron
B) calcium
C) iodine
D) sodium
E) potassium

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
18
The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons?
A) 6
B) 7
C) 8
D) 12
E) 14
A) 6
B) 7
C) 8
D) 12
E) 14

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
19
Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A) 1
B) 3
C) 0
D) 7
E) 9
A) 1
B) 3
C) 0
D) 7
E) 9

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
20
An atom with atomic number 12 would have what type of chemical behavior in bonding with other elements?
A) It would form ions with a +1 charge.
B) It would form ions with a +2 charge.
C) It would form ions with a -1 charge.
D) It would form ions with a -2 charge.
E) It would form two covalent bonds with other atoms.
A) It would form ions with a +1 charge.
B) It would form ions with a +2 charge.
C) It would form ions with a -1 charge.
D) It would form ions with a -2 charge.
E) It would form two covalent bonds with other atoms.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
21
When two atoms are equally electronegative, they will interact to form
A) hydrogen bonds.
B) van der Waals interactions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.
A) hydrogen bonds.
B) van der Waals interactions.
C) polar covalent bonds.
D) nonpolar covalent bonds.
E) ionic bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
22
If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1), which of the following molecules would be formed?
A) S-H
B) H-S-H
C)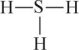
D)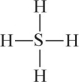
E) H = S = H
A) S-H
B) H-S-H
C)
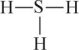
D)

E) H = S = H

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
23
An ionic bond is formed by
A) sharing of a pair of electrons between two atoms.
B) sharing of a single electron between two atoms.
C) sharing of a pair of protons between two atoms.
D) transfer of an electron from one atom to another.
E) transfer of a proton from one atom to another.
A) sharing of a pair of electrons between two atoms.
B) sharing of a single electron between two atoms.
C) sharing of a pair of protons between two atoms.
D) transfer of an electron from one atom to another.
E) transfer of a proton from one atom to another.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following are the strongest molecular interactions?
A) van der Waals interactions
B) van der Waals interactions in a nonpolar environment
C) ionic bonds in an aqueous environment
D) covalent bonds
E) hydrogen bonds
A) van der Waals interactions
B) van der Waals interactions in a nonpolar environment
C) ionic bonds in an aqueous environment
D) covalent bonds
E) hydrogen bonds

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
25
Sulfur (atomic number 16) will have chemical properties most similar to
A) carbon (atomic number 6).
B) nitrogen (atomic number 7).
C) oxygen (atomic number 8).
D) phosphorous (atomic number 15).
E) chlorine (atomic number 17).
A) carbon (atomic number 6).
B) nitrogen (atomic number 7).
C) oxygen (atomic number 8).
D) phosphorous (atomic number 15).
E) chlorine (atomic number 17).

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
26
In ammonium chloride salt (NH4Cl), the anion is a single chloride ion, Cl. What is the cation of NH4Cl?
A) N, with a charge of +1
B) NH, with a charge of +1
C) H3, with a charge of +1
D) NH4, with a charge of +1
E) NH4, with a charge of +4
A) N, with a charge of +1
B) NH, with a charge of +1
C) H3, with a charge of +1
D) NH4, with a charge of +1
E) NH4, with a charge of +4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
27
A covalent chemical bond is one in which
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C) outer-shell electrons of two atoms are shared so as to occupy the outer electron shells of both atoms.
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom.
E) an electron from a full outer electron shell of one atom is shared so as to occupy the outer electron shell of both atoms.
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged.
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms.
C) outer-shell electrons of two atoms are shared so as to occupy the outer electron shells of both atoms.
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom.
E) an electron from a full outer electron shell of one atom is shared so as to occupy the outer electron shell of both atoms.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
28
A covalent bond is formed by
A) sharing of a pair of electrons between two atoms.
B) sharing of a single electron between two atoms.
C) sharing of a pair of protons between two atoms.
D) transfer of an electron from one atom to another.
E) transfer of a proton from one atom to another.
A) sharing of a pair of electrons between two atoms.
B) sharing of a single electron between two atoms.
C) sharing of a pair of protons between two atoms.
D) transfer of an electron from one atom to another.
E) transfer of a proton from one atom to another.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
29
What results from an unequal sharing of electrons between atoms?
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrophobic interaction
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrophobic interaction

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
30
What type of bonding or interaction is most likely to occur among a broad array of molecules with various physical properties (polar, nonpolar, hydrophilic, hydrophobic)?
A) covalent bonding
B) polar covalent bonding
C) ionic bonding
D) hydrogen bonding
E) van der Waals interactions
A) covalent bonding
B) polar covalent bonding
C) ionic bonding
D) hydrogen bonding
E) van der Waals interactions

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
31
What is the maximum number of covalent bonds an element with atomic number 8 can make with hydrogen?
A) 1
B) 2
C) 3
D) 4
E) 6
A) 1
B) 2
C) 3
D) 4
E) 6

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
32
Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements about the atoms in ammonia (NH3) is correct?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) The nitrogen atom has a full positive charge; each hydrogen atom has a full positive charge.
C) Each hydrogen atom has a partial negative charge; the nitrogen atom has a full positive charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
E) There are nonpolar covalent bonds between the hydrogen atoms and polar covalent bonds between each hydrogen atom and the nitrogen atom.
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) The nitrogen atom has a full positive charge; each hydrogen atom has a full positive charge.
C) Each hydrogen atom has a partial negative charge; the nitrogen atom has a full positive charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
E) There are nonpolar covalent bonds between the hydrogen atoms and polar covalent bonds between each hydrogen atom and the nitrogen atom.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
33
The most stable interaction between magnesium (atomic number 12) and chlorine (atomic number 17) forms
A) MgCl, in which atoms are joined by covalent bonds.
B) MgCl, in which atoms are joined by ionic bonds.
C) Mg2Cl, in which atoms are joined by ionic bonds.
D) MgCl2, in which atoms are joined by covalent bonds.
E) MgCl2, in which atoms are joined by ionic bonds.
A) MgCl, in which atoms are joined by covalent bonds.
B) MgCl, in which atoms are joined by ionic bonds.
C) Mg2Cl, in which atoms are joined by ionic bonds.
D) MgCl2, in which atoms are joined by covalent bonds.
E) MgCl2, in which atoms are joined by ionic bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
34
Which bond or interaction would be most difficult to disrupt when compounds are put into water?
A) covalent bond
B) hydrogen bond
C) van der Waals interaction
D) ionic bond
A) covalent bond
B) hydrogen bond
C) van der Waals interaction
D) ionic bond

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following molecules contains the most polar covalent bond?
A) H2
B) O₂
C) CO₂
D) H₂O
E) CH4
A) H2
B) O₂
C) CO₂
D) H₂O
E) CH4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
36
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
37
A covalent bond is likely to be polar when
A) one of the atoms sharing electrons is much more electronegative than the other atom.
B) the two atoms sharing electrons are equally electronegative.
C) oxygen is one of the two atoms sharing electrons.
D) the two atoms sharing electrons are the same element.
E) the two atoms sharing electrons are different elements.
A) one of the atoms sharing electrons is much more electronegative than the other atom.
B) the two atoms sharing electrons are equally electronegative.
C) oxygen is one of the two atoms sharing electrons.
D) the two atoms sharing electrons are the same element.
E) the two atoms sharing electrons are different elements.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
38
The atomic number of chlorine is 17. The atomic number of calcium is 20. What is the chemical formula for calcium chloride?
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
39
How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H6?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
40
How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H4?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
41
How many molecules of glycerol (C3H8O3; molecular mass = 92) are present in 1 L of a 0.5 M glycerol solution?
A) 1 × 1023
B) 0.5 × 6.02 × 1023
C) 92/2 × 6.02 × 1023
D) 0.5 × 6.02/92 × 1023
E) 6.02 × 1023
A) 1 × 1023
B) 0.5 × 6.02 × 1023
C) 92/2 × 6.02 × 1023
D) 0.5 × 6.02/92 × 1023
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
42
Water molecules are able to form hydrogen bonds with
A) compounds that are not soluble in water.
B) compounds that have nonpolar covalent bonds.
C) oxygen gas (O₂) molecules.
D) methane gas (CH4) molecules.
E) compounds that have polar covalent bonds.
A) compounds that are not soluble in water.
B) compounds that have nonpolar covalent bonds.
C) oxygen gas (O₂) molecules.
D) methane gas (CH4) molecules.
E) compounds that have polar covalent bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
43
The slight negative charge at one end of one water molecule is attracted to the slight positive charge of another water molecule. What is this attraction called?
A) a covalent bond
B) a hydrogen bond
C) an ionic bond
D) a hydrophilic bond
E) a van der Waals interaction
A) a covalent bond
B) a hydrogen bond
C) an ionic bond
D) a hydrophilic bond
E) a van der Waals interaction

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following correctly describes any reaction that has reached chemical equilibrium?
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.
A) The concentration of the reactants equals the concentration of the products.
B) The rate of the forward reaction is equal to the rate of the reverse reaction.
C) All of the reactants have been converted to the products of the reaction.
D) All of the products have been converted to the reactants of the reaction.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
45
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
A) 2
B) 3
C) 4
D) 6
E) 8
A) 2
B) 3
C) 4
D) 6
E) 8

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
46
Sulfur is in the same column of the periodic table as oxygen but has electronegativity similar to carbon. Compared to water molecules, molecules of H2S will
A) ionize more readily.
B) have greater cohesion to other molecules of H2S.
C) have a greater tendency to form hydrogen bonds with each other.
D) have a higher capacity to absorb heat for the same change in temperature.
E) not form hydrogen bonds with each other.
A) ionize more readily.
B) have greater cohesion to other molecules of H2S.
C) have a greater tendency to form hydrogen bonds with each other.
D) have a higher capacity to absorb heat for the same change in temperature.
E) not form hydrogen bonds with each other.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
47
Glucose has a molecular mass of 180 g/mol. How many glucose molecules are present in 90 grams of glucose?
A) 90 × 1023
B) (6.02/180) × 1023
C) (6.02/90) × 1023
D) (90 × 6.02) × 1023
E) (90/180) × 6.02 × 1023
A) 90 × 1023
B) (6.02/180) × 1023
C) (6.02/90) × 1023
D) (90 × 6.02) × 1023
E) (90/180) × 6.02 × 1023

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following are considered compounds?
A) H₂O, O₂, and CH4
B) H₂O and O₂
C) O₂ and CH4
D) CH4 and O₂, but not H₂O
E) H₂O and CH4, but not O₂
A) H₂O, O₂, and CH4
B) H₂O and O₂
C) O₂ and CH4
D) CH4 and O₂, but not H₂O
E) H₂O and CH4, but not O₂

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
49
Hydrophobic substances such as vegetable oil are
A) nonpolar substances that repel water molecules.
B) nonpolar substances that have an attraction for water molecules.
C) polar substances that repel water molecules.
D) polar substances that have an affinity for water.
E) charged molecules that hydrogen-bond with water molecules.
A) nonpolar substances that repel water molecules.
B) nonpolar substances that have an attraction for water molecules.
C) polar substances that repel water molecules.
D) polar substances that have an affinity for water.
E) charged molecules that hydrogen-bond with water molecules.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
50
In an aqueous solution, water molecules associate with one another through which of the following?
A) hydrogen bonds
B) ionic bonds
C) polar covalent bonds
D) covalent bonds
E) hydrophobic interaction
A) hydrogen bonds
B) ionic bonds
C) polar covalent bonds
D) covalent bonds
E) hydrophobic interaction

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
51
One mole (mol) of glucose (molecular mass = 180 daltons) is
A) 180 × 1023 molecules of glucose.
B) 1 kg of glucose dissolved in 1 L of solution.
C) the largest amount of glucose that can be dissolved in 1 L of solution.
D) 180 grams of glucose.
E) 180 grams of glucose dissolved in 1 L of solution.
A) 180 × 1023 molecules of glucose.
B) 1 kg of glucose dissolved in 1 L of solution.
C) the largest amount of glucose that can be dissolved in 1 L of solution.
D) 180 grams of glucose.
E) 180 grams of glucose dissolved in 1 L of solution.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following effects is produced by the high surface tension of water?
A) Lakes don't freeze solid in winter despite low temperatures.
B) A water strider can walk across the surface of a small pond.
C) Organisms resist temperature changes, although they give off heat due to chemical reactions.
D) Evaporation of sweat from the skin helps to keep people from overheating.
E) Water flows upward from the roots to the leaves in plants.
A) Lakes don't freeze solid in winter despite low temperatures.
B) A water strider can walk across the surface of a small pond.
C) Organisms resist temperature changes, although they give off heat due to chemical reactions.
D) Evaporation of sweat from the skin helps to keep people from overheating.
E) Water flows upward from the roots to the leaves in plants.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
53
The nutritional information on a cereal box shows that one serving of a dry cereal has 200 kilocalories. If a person were to ignite one serving of the cereal in a bowl, the amount of heat given off would be sufficient to raise the temperature of 20 kg of water how many degrees Celsius?
A) 0.2°C
B) 1.0°C
C) 2.0°C
D) 10.0°C
E) 20.0°C
A) 0.2°C
B) 1.0°C
C) 2.0°C
D) 10.0°C
E) 20.0°C

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following takes place as an ice cube cools a drink?
A) Molecular collisions in the drink increase.
B) Kinetic energy in the drink decreases.
C) A calorie of heat energy is transferred from the ice to the water of the drink.
D) The specific heat of the water in the drink decreases.
E) Evaporation of the water in the drink increases.
A) Molecular collisions in the drink increase.
B) Kinetic energy in the drink decreases.
C) A calorie of heat energy is transferred from the ice to the water of the drink.
D) The specific heat of the water in the drink decreases.
E) Evaporation of the water in the drink increases.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B) The concentrations of the products are higher than the concentrations of the reactants.
C) Forward and reverse reactions have stopped so that the concentrations of the reactants and products remain constant.
D) Reactions stop only when all reactants have been converted to products.
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B) The concentrations of the products are higher than the concentrations of the reactants.
C) Forward and reverse reactions have stopped so that the concentrations of the reactants and products remain constant.
D) Reactions stop only when all reactants have been converted to products.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
56
If a salamander clings to surfaces through hydrogen bonds, it would have the most difficulty clinging to which of the following surfaces?
A) a surface coated with a thin film of water
B) a surface coated with a thin film of vinegar (acetic acid)
C) a surface coated with a thin film of vegetable oil
D) a surface coated with a thin film of ammonia (NH3)
A) a surface coated with a thin film of water
B) a surface coated with a thin film of vinegar (acetic acid)
C) a surface coated with a thin film of vegetable oil
D) a surface coated with a thin film of ammonia (NH3)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
57
Which type of bond must be broken for water to vaporize?
A) ionic bonds
B) both hydrogen bonds and ionic bonds
C) polar covalent bonds
D) hydrogen bonds
E) both polar covalent bonds and hydrogen bonds
A) ionic bonds
B) both hydrogen bonds and ionic bonds
C) polar covalent bonds
D) hydrogen bonds
E) both polar covalent bonds and hydrogen bonds

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
58
A dietary Calorie equals 1 kilocalorie. Which of the following statements correctly defines 1 kilocalorie?
A) 1,000 calories, or the amount of heat required to raise the temperature of 1 g of water by 1,000°C
B) 100 calories, or the amount of heat required to raise the temperature of 100 g of water by 1°C
C) 10,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°F
D) 1,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°C
E) 1,000 calories, or the amount of heat required to raise the temperature of 100 g of water by 100°C
A) 1,000 calories, or the amount of heat required to raise the temperature of 1 g of water by 1,000°C
B) 100 calories, or the amount of heat required to raise the temperature of 100 g of water by 1°C
C) 10,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°F
D) 1,000 calories, or the amount of heat required to raise the temperature of 1 kg of water by 1°C
E) 1,000 calories, or the amount of heat required to raise the temperature of 100 g of water by 100°C

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
59
In a single molecule of water, two hydrogen atoms are bonded to a single oxygen atom by
A) hydrogen bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds.
D) ionic bonds.
E) van der Waals interactions.
A) hydrogen bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds.
D) ionic bonds.
E) van der Waals interactions.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
60
Why does ice float in liquid water?
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Ice always has air bubbles that keep it afloat.
D) Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water.
E) The crystalline lattice of ice causes it to be denser than liquid water.
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Ice always has air bubbles that keep it afloat.
D) Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water.
E) The crystalline lattice of ice causes it to be denser than liquid water.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
61
What is the hydrogen ion (H+) concentration of a solution of pH 8?
A) 8 M
B) 8 × 10-6 M
C) 0.01 M
D) 10-8 M
E) 10-6 M
A) 8 M
B) 8 × 10-6 M
C) 0.01 M
D) 10-8 M
E) 10-6 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
62
How many glucose molecules are contained in 1 liter of a 10 M solution of glucose in water?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
63
How many glucose molecules are contained in 0.1 liter of a 10 M solution of glucose in water?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
64
You have a freshly prepared 1 M solution of glucose in water. You carefully pour out a 100-mL sample of that solution. How many glucose molecules are included in that 100-mL sample?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
65
What is the pH of a solution with a hydroxyl ion (OH-) concentration of 10-12 M?
A) pH 2
B) pH 4
C) pH 10
D) pH 12
E) pH 14
A) pH 2
B) pH 4
C) pH 10
D) pH 12
E) pH 14

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
66
A solution contains 0.0000001(10-7) moles of hydroxyl ions (OH-) per liter. Which of the following best describes this solution?
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
67
The molar mass of water is 18 g/mol. What is the molarity of 1 liter of pure water? (Hint: One liter of pure water has a mass of 1 kg.)
A) 55.6 M
B) 18 M
C) 37 M
D) 0.66 M
E) 1.0 M
A) 55.6 M
B) 18 M
C) 37 M
D) 0.66 M
E) 1.0 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following solutions would require the greatest amount of base to be added to bring the solution to neutral pH?
A) gastric juice at pH 2
B) vinegar at pH 3
C) tomato juice at pH 4
D) black coffee at pH 5
E) household bleach at pH 12
A) gastric juice at pH 2
B) vinegar at pH 3
C) tomato juice at pH 4
D) black coffee at pH 5
E) household bleach at pH 12

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
69
How many molecules of glycerol (C3H8O3; molecular mass = 92) are present in 0.5 L of a 1 M glycerol solution?
A) 1 × 1023
B) 0.5 × 6.02 × 1023
C) 92/2 × 6.02 × 1023
D) 0.5 × 6.02/92 × 1023
E) 6.02 × 1023
A) 1 × 1023
B) 0.5 × 6.02 × 1023
C) 92/2 × 6.02 × 1023
D) 0.5 × 6.02/92 × 1023
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
70
A strong acid like HCl
A) dissociates completely in an aqueous solution.
B) increases the pH when added to an aqueous solution.
C) reacts with strong bases to create a buffered solution.
D) is a strong buffer at low pH.
E) is a strong buffer at high pH.
A) dissociates completely in an aqueous solution.
B) increases the pH when added to an aqueous solution.
C) reacts with strong bases to create a buffered solution.
D) is a strong buffer at low pH.
E) is a strong buffer at high pH.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
71
What is the pH of a 1-millimolar NaOH solution?
A) pH 3
B) pH 8
C) pH 9
D) pH 10
E) pH 11
A) pH 3
B) pH 8
C) pH 9
D) pH 10
E) pH 11

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
72
If the pH of a solution is decreased from pH 8 to pH 6, it means that the
A) concentration of H+ is twice (2×) what it was at pH 8.
B) concentration of H+ is one-half (1/2) what it was at pH 8.
C) concentration of OH- is 100 times greater than what it was at pH 8.
D) concentration of OH- is one-hundredth (0.01×) what it was at pH 8.
E) concentration of H+ is one-hundredth (0.01×) what it was at pH 8.
A) concentration of H+ is twice (2×) what it was at pH 8.
B) concentration of H+ is one-half (1/2) what it was at pH 8.
C) concentration of OH- is 100 times greater than what it was at pH 8.
D) concentration of OH- is one-hundredth (0.01×) what it was at pH 8.
E) concentration of H+ is one-hundredth (0.01×) what it was at pH 8.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
73
A 0.01 M solution of a substance has a pH of 2. What can you conclude about this substance?
A) It is a strong acid that ionizes completely in water.
B) It is a strong base that ionizes completely in water.
C) It is a weak acid.
D) It is a weak base.
E) It is a buffer.
A) It is a strong acid that ionizes completely in water.
B) It is a strong base that ionizes completely in water.
C) It is a weak acid.
D) It is a weak base.
E) It is a buffer.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
74
What is the hydroxyl ion (OH-) concentration of a solution of pH 8?
A) 8 M
B) 8 × 10-6 M
C) 0.01 M
D) 10-8 M
E) 10-6 M
A) 8 M
B) 8 × 10-6 M
C) 0.01 M
D) 10-8 M
E) 10-6 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
75
How many glucose molecules are contained in one liter of a 0.1 M solution of glucose in water?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
76
If the pH of a solution is increased from pH 5 to pH 7, it means that the
A) concentration of H+ is twice (2×) what it was at pH 5.
B) concentration of H+ is one-half (1/2) what it was at pH 5.
C) concentration of OH- is 100 times greater than what it was at pH 5.
D) concentration of OH- is one-hundredth (0.01×) what it was at pH 5.
E) concentration of H+ is 100 times greater than what it was at pH 5.
A) concentration of H+ is twice (2×) what it was at pH 5.
B) concentration of H+ is one-half (1/2) what it was at pH 5.
C) concentration of OH- is 100 times greater than what it was at pH 5.
D) concentration of OH- is one-hundredth (0.01×) what it was at pH 5.
E) concentration of H+ is 100 times greater than what it was at pH 5.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
77
When an ionic compound such as sodium chloride (NaCl) is placed in water, the component atoms of the NaCl crystal dissociate into individual sodium ions (Na+) and chloride ions (Cl-). In contrast, the atoms of covalently bonded molecules (e.g., glucose, sucrose, glycerol) do not generally dissociate when placed in aqueous solution. Which of the following solutions would be expected to contain the greatest number of solute particles (molecules or ions)?
A) 1 L of 0.5 M NaCl
B) 1 L of 0.5 M glucose
C) 1 L of 1.0 M NaCl
D) 1 L of 1.0 M glucose
E) 2 L of 0.5 M glucose.
A) 1 L of 0.5 M NaCl
B) 1 L of 0.5 M glucose
C) 1 L of 1.0 M NaCl
D) 1 L of 1.0 M glucose
E) 2 L of 0.5 M glucose.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
78
The molar mass of glucose (C₆H₁₂O₆) is 180 g/mol. Which of the following procedures should you carry out to make a 0.5 M solution of glucose?
A) Dissolve 0.5 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
B) Dissolve 90 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
C) Dissolve 180 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
D) Dissolve 0.5 g of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.5 L of water.
A) Dissolve 0.5 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
B) Dissolve 90 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
C) Dissolve 180 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
D) Dissolve 0.5 g of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.5 L of water.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
79
The molar mass of glucose is 180 g/mol. Which of the following procedures should you carry out to make a 1 M solution of glucose?
A) Dissolve 1 g of glucose in 1 L of water.
B) Dissolve 180 g of glucose in 1 L of water.
C) Dissolve 180 g of glucose in 180 g of water.
D) Dissolve 180 milligrams (mg) of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.8 L of water, and then add more water until the total volume of the solution is 1 L.
A) Dissolve 1 g of glucose in 1 L of water.
B) Dissolve 180 g of glucose in 1 L of water.
C) Dissolve 180 g of glucose in 180 g of water.
D) Dissolve 180 milligrams (mg) of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.8 L of water, and then add more water until the total volume of the solution is 1 L.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following dissociates completely in aqueous solution and is therefore considered to be a strong base (alkali)?
A) NaCl
B) HCl
C) NH3
D) H2CO3
E) NaOH
A) NaCl
B) HCl
C) NH3
D) H2CO3
E) NaOH

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck


