Deck 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make
1
The forward rate constant, kf, and reverse rate constant, kr, for a chemical reaction are not equal. Which of the following must be true?
A)The reaction will be unable to achieve equilibrium.
B)kf and kr will become equal as equilibrium is approached, owing to concentration changes.
C)kf and kr will become equal as equilibrium is approached, owing to temperature changes.
D)kf and kr will remain unequal but the rates will become equal, owing to concentration changes.
E)kf and kr will remain unequal but the rates will become equal, owing to temperature changes.
A)The reaction will be unable to achieve equilibrium.
B)kf and kr will become equal as equilibrium is approached, owing to concentration changes.
C)kf and kr will become equal as equilibrium is approached, owing to temperature changes.
D)kf and kr will remain unequal but the rates will become equal, owing to concentration changes.
E)kf and kr will remain unequal but the rates will become equal, owing to temperature changes.
kf and kr will remain unequal but the rates will become equal, owing to concentration changes.
2
A chemical equilibrium 2 A 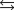 B
B
Has a forward rate constant, kf 10 M 1s1, and a reverse rate constant, kr 5.0 s1. What is the value of the equilibrium constant for this system?
A)0.5
B)2.0
C)20
D)0.050
E)5.0
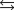 B
BHas a forward rate constant, kf 10 M 1s1, and a reverse rate constant, kr 5.0 s1. What is the value of the equilibrium constant for this system?
A)0.5
B)2.0
C)20
D)0.050
E)5.0
2.0
3
For an equilibrium reaction with K 4.3 103, the reverse rate constant was found to be 2.1 102. What is the value of the forward rate constant?
A)2.0 105
B)3.1 103
C)9.0 101
D)4.9 104
E)5.3 103
A)2.0 105
B)3.1 103
C)9.0 101
D)4.9 104
E)5.3 103
9.0 101
4
A chemical equilibrium 2 A ![<strong>A chemical equilibrium 2<sub> </sub>A B Has a forward rate constant, k<sub>f </sub><font face=symbol></font> 10 M <font face=symbol><sup></sup></font><sup>1 </sup>s<font face=symbol><sup></sup></font><sup>1</sup>, and a reverse rate constant, k<sub>r</sub> <font face=symbol></font> 5 s<font face=symbol><sup></sup></font><sup>1</sup>. If the system has a concentration of [A] <font face=symbol></font> 0.10 M at equilibrium, what must be the concentration of B at equilibrium?</strong> A)20.0 B)2.0 C)0.20 D)0.020 E)200](https://storage.examlex.com/TB3835/11eaa952_9b4e_b3be_ae90_0d83087e18c5_TB3835_11.jpg) B
B
Has a forward rate constant, kf 10 M 1 s1, and a reverse rate constant, kr 5 s1. If the system has a concentration of [A] 0.10 M at equilibrium, what must be the concentration of B at equilibrium?
A)20.0
B)2.0
C)0.20
D)0.020
E)200
![<strong>A chemical equilibrium 2<sub> </sub>A B Has a forward rate constant, k<sub>f </sub><font face=symbol></font> 10 M <font face=symbol><sup></sup></font><sup>1 </sup>s<font face=symbol><sup></sup></font><sup>1</sup>, and a reverse rate constant, k<sub>r</sub> <font face=symbol></font> 5 s<font face=symbol><sup></sup></font><sup>1</sup>. If the system has a concentration of [A] <font face=symbol></font> 0.10 M at equilibrium, what must be the concentration of B at equilibrium?</strong> A)20.0 B)2.0 C)0.20 D)0.020 E)200](https://storage.examlex.com/TB3835/11eaa952_9b4e_b3be_ae90_0d83087e18c5_TB3835_11.jpg) B
BHas a forward rate constant, kf 10 M 1 s1, and a reverse rate constant, kr 5 s1. If the system has a concentration of [A] 0.10 M at equilibrium, what must be the concentration of B at equilibrium?
A)20.0
B)2.0
C)0.20
D)0.020
E)200

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
A chemical equilibrium may be established by starting a reaction with ________
A)reactants only.
B)products only.
C)equal quantities of reactants and products.
D)any quantities of reactants and products.
E)all of the above.
A)reactants only.
B)products only.
C)equal quantities of reactants and products.
D)any quantities of reactants and products.
E)all of the above.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
Which expression corresponds to the equilibrium constant for the reaction written as 2H2(g) O2(g) ![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_4be5_ae90_8ff1a3473ea6_TB3835_11.jpg) 2H2O2(g)?
2H2O2(g)?
A)[H2]2[O2]
B)![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f6_ae90_2f7e4ff24fd7_TB3835_11.jpg)
C)![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f7_ae90_e958dd5b7864_TB3835_11.jpg)
D)![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f8_ae90_31b6317393fd_TB3835_11.jpg)
E)![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_9a09_ae90_d945061b9787_TB3835_11.jpg)
![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_4be5_ae90_8ff1a3473ea6_TB3835_11.jpg) 2H2O2(g)?
2H2O2(g)?A)[H2]2[O2]
B)
![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f6_ae90_2f7e4ff24fd7_TB3835_11.jpg)
C)
![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f7_ae90_e958dd5b7864_TB3835_11.jpg)
D)
![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_72f8_ae90_31b6317393fd_TB3835_11.jpg)
E)
![<strong>Which expression corresponds to the equilibrium constant for the reaction written as 2H<sub>2</sub>(g) <font face=symbol></font> O<sub>2</sub>(g) 2H<sub>2</sub>O<sub>2</sub>(g)?</strong> A)[H<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b51_9a09_ae90_d945061b9787_TB3835_11.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement about a chemical reaction is not correct?
A)All chemical reactions are reversible since products can be converted back into the reactants.
B)If a reaction is characterized as lying far to the left, then the concentration of products is small when equilibrium is reached.
C)At equilibrium, the rates of the forward reaction and reverse reaction are equal.
D)At equilibrium, the rates of the forward and reverse reactions are equal because both have stopped.
E)Chemical equilibrium is said to be "a dynamic process."
A)All chemical reactions are reversible since products can be converted back into the reactants.
B)If a reaction is characterized as lying far to the left, then the concentration of products is small when equilibrium is reached.
C)At equilibrium, the rates of the forward reaction and reverse reaction are equal.
D)At equilibrium, the rates of the forward and reverse reactions are equal because both have stopped.
E)Chemical equilibrium is said to be "a dynamic process."

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
In a reversible reaction A ![<strong>In a reversible reaction A B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?</strong> A)Can't tell, need more information. B)[A] <font face=symbol></font> [B] C)[A] <font face=symbol></font> 2 <font face=symbol></font> [B] D)[A] <font face=symbol></font><font face=symbol></font> <font face=symbol></font> [B] E)[A] <font face=symbol></font> 4 <font face=symbol></font> [B]](https://storage.examlex.com/TB3835/11eaa952_9b4e_dad0_ae90_453eb2242066_TB3835_11.jpg) B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?
B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?
A)Can't tell, need more information.
B)[A] [B]
C)[A] 2 [B]
D)[A] ![<strong>In a reversible reaction A B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?</strong> A)Can't tell, need more information. B)[A] <font face=symbol></font> [B] C)[A] <font face=symbol></font> 2 <font face=symbol></font> [B] D)[A] <font face=symbol></font><font face=symbol></font> <font face=symbol></font> [B] E)[A] <font face=symbol></font> 4 <font face=symbol></font> [B]](https://storage.examlex.com/TB3835/11eaa952_9b4f_01e1_ae90_39ff5042d7d9_TB3835_11.jpg) [B]
[B]
E)[A] 4 [B]
![<strong>In a reversible reaction A B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?</strong> A)Can't tell, need more information. B)[A] <font face=symbol></font> [B] C)[A] <font face=symbol></font> 2 <font face=symbol></font> [B] D)[A] <font face=symbol></font><font face=symbol></font> <font face=symbol></font> [B] E)[A] <font face=symbol></font> 4 <font face=symbol></font> [B]](https://storage.examlex.com/TB3835/11eaa952_9b4e_dad0_ae90_453eb2242066_TB3835_11.jpg) B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?
B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?A)Can't tell, need more information.
B)[A] [B]
C)[A] 2 [B]
D)[A]
![<strong>In a reversible reaction A B at a particular temperature, the rate constant for the reverse reaction is twice the rate constant for the forward reaction. At equilibrium, how are the concentrations of A and B related?</strong> A)Can't tell, need more information. B)[A] <font face=symbol></font> [B] C)[A] <font face=symbol></font> 2 <font face=symbol></font> [B] D)[A] <font face=symbol></font><font face=symbol></font> <font face=symbol></font> [B] E)[A] <font face=symbol></font> 4 <font face=symbol></font> [B]](https://storage.examlex.com/TB3835/11eaa952_9b4f_01e1_ae90_39ff5042d7d9_TB3835_11.jpg) [B]
[B]E)[A] 4 [B]

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
Write the equilibrium expression for the reaction 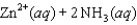
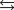
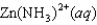
A)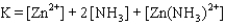
B)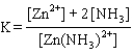
C)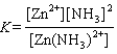
D)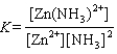
E)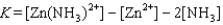
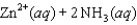
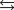
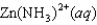
A)
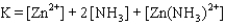
B)
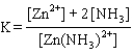
C)
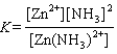
D)
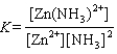
E)
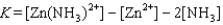

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
A chemical equilibrium A  3B
3B
Has a forward rate constant, s1 5 M 1s1, and a reverse rate constant, M 2s1 0.5 s1. What is the value of the equilibrium constant for this system?
A)0.1
B)0.5
C)5.0
D)10
E)15
 3B
3BHas a forward rate constant, s1 5 M 1s1, and a reverse rate constant, M 2s1 0.5 s1. What is the value of the equilibrium constant for this system?
A)0.1
B)0.5
C)5.0
D)10
E)15

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
The law of mass action is a result of ________
A)the law of conservation of matter.
B)the law of conservation of energy.
C)kinetics of reversible reactions.
D)limiting reactant stoichiometry.
E)the third law of thermodynamics.
A)the law of conservation of matter.
B)the law of conservation of energy.
C)kinetics of reversible reactions.
D)limiting reactant stoichiometry.
E)the third law of thermodynamics.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following can be predicted from the law of mass action?
I. At equilibrium, the ratio of concentrations of products to reactants, each raised to a power corresponding to the stoichiometric coefficient in the balanced reaction equation, will have a constant value at a given temperature.
II. The direction of the reaction given values for the reactant and product concentrations and the equilibrium constant.
III. The amounts of products that can be produced from a given amount of reactants.
A)I only
B)II and III
C)I, II, and III
D)I and II
E)I and III
I. At equilibrium, the ratio of concentrations of products to reactants, each raised to a power corresponding to the stoichiometric coefficient in the balanced reaction equation, will have a constant value at a given temperature.
II. The direction of the reaction given values for the reactant and product concentrations and the equilibrium constant.
III. The amounts of products that can be produced from a given amount of reactants.
A)I only
B)II and III
C)I, II, and III
D)I and II
E)I and III

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is true for a chemical reaction at equilibrium regarding the concentration of products?
A)They will not change because there are no more reactants.
B)They will not change because the limiting reagent is gone.
C)They will not change because this is a constant for each reaction.
D)They will not change because the forward and reverse rates are equal.
E)They will change continually because of reversibility.
A)They will not change because there are no more reactants.
B)They will not change because the limiting reagent is gone.
C)They will not change because this is a constant for each reaction.
D)They will not change because the forward and reverse rates are equal.
E)They will change continually because of reversibility.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
For an equilibrium reaction with K 1.2 108, the forward rate constant was found to be 3.5 105. What is the value of the reverse rate constant?
A)3.5 105
B)3.4 102
C)4.2 1013
D)2.9 103
E)6.0 105
A)3.5 105
B)3.4 102
C)4.2 1013
D)2.9 103
E)6.0 105

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is true for a chemical reaction at equilibrium?
A)Only the forward reaction stops.
B)Only the reverse reaction stops.
C)Both the forward and reverse reactions stop.
D)The rate constants for the forward and reverse reactions are equal.
E)The rates of the forward and reverse reactions are equal.
A)Only the forward reaction stops.
B)Only the reverse reaction stops.
C)Both the forward and reverse reactions stop.
D)The rate constants for the forward and reverse reactions are equal.
E)The rates of the forward and reverse reactions are equal.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is true for a chemical reaction at equilibrium?
A)All reaction has ceased.
B)The reaction has gone to completion to products.
C)The amount of reactant(s) remaining is always equal to the amount of product(s) formed.
D)The rates of the forward and reverse reactions are equal.
E)The concentrations of products and reactants are still changing.
A)All reaction has ceased.
B)The reaction has gone to completion to products.
C)The amount of reactant(s) remaining is always equal to the amount of product(s) formed.
D)The rates of the forward and reverse reactions are equal.
E)The concentrations of products and reactants are still changing.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
Which of A-D is equal in an equilibrium?
A)the concentrations of reactants and products
B)the rate constants for the forward and reverse reactions
C)the time that a particular atom or molecule spends as a reactant and product
D)the rate of the forward and reverse reaction
E)All of the above are equal.
A)the concentrations of reactants and products
B)the rate constants for the forward and reverse reactions
C)the time that a particular atom or molecule spends as a reactant and product
D)the rate of the forward and reverse reaction
E)All of the above are equal.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
In a reversible reaction A  B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?
B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?
A)Can't tell, need more information.
B)0.25
C)4
D)16
E)0.5
 B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?
B at a particular temperature, the rate constant for the reverse reaction is 4 times the rate constant for the forward reaction. What is the value of the equilibrium constant at that temperature?A)Can't tell, need more information.
B)0.25
C)4
D)16
E)0.5

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the equilibrium A B ![<strong>Consider the equilibrium A <font face=symbol></font> B C. What is significant about the equilibrium state in which [B] <font face=symbol></font> [C]?</strong> A)[A] <font face=symbol></font> K B)[A] <font face=symbol></font> [B] <font face=symbol></font> [C] C)[B] <font face=symbol></font> [C] <font face=symbol></font> K D)[A] <font face=symbol></font> 1/K E)[C]/[B] <font face=symbol></font> K](https://storage.examlex.com/TB3835/11eaa952_9b50_888c_ae90_8b3c2537470e_TB3835_11.jpg) C. What is significant about the equilibrium state in which [B] [C]?
C. What is significant about the equilibrium state in which [B] [C]?
A)[A] K
B)[A] [B] [C]
C)[B] [C] K
D)[A] 1/K
E)[C]/[B] K
![<strong>Consider the equilibrium A <font face=symbol></font> B C. What is significant about the equilibrium state in which [B] <font face=symbol></font> [C]?</strong> A)[A] <font face=symbol></font> K B)[A] <font face=symbol></font> [B] <font face=symbol></font> [C] C)[B] <font face=symbol></font> [C] <font face=symbol></font> K D)[A] <font face=symbol></font> 1/K E)[C]/[B] <font face=symbol></font> K](https://storage.examlex.com/TB3835/11eaa952_9b50_888c_ae90_8b3c2537470e_TB3835_11.jpg) C. What is significant about the equilibrium state in which [B] [C]?
C. What is significant about the equilibrium state in which [B] [C]?A)[A] K
B)[A] [B] [C]
C)[B] [C] K
D)[A] 1/K
E)[C]/[B] K

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
At a high temperature, carbon dioxide decomposes to produce carbon monoxide and oxygen. Which expression corresponds to the equilibrium constant for the reaction written as 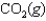
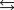
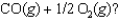
A)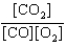
B)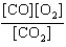
C)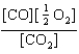
D)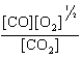
E)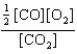
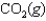
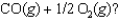
A)
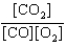
B)
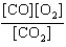
C)
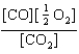
D)
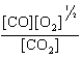
E)
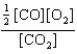

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate K for the following reaction, provided the concentration versus time graph shown below. 2A  2B 3C
2B 3C 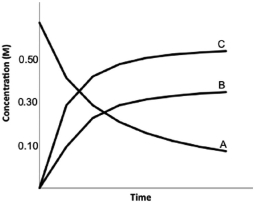
A)0.50
B)0.68
C)1.1
D)1.5
E)2.8
 2B 3C
2B 3C 
A)0.50
B)0.68
C)1.1
D)1.5
E)2.8

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
An equilibrium that strongly favors products has ________
A)a value of K 1.
B)a value of K 1.
C)a value of Q 1.
D)a value of Q 1.
E)K Q.
A)a value of K 1.
B)a value of K 1.
C)a value of Q 1.
D)a value of Q 1.
E)K Q.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
A decrease in the number of moles of gas as the reaction goes from reactants to products in a gas-phase equilibrium results in ________
A)Kp Kc.
B)Kp Kc.
C)Kp Kc.
D)Kp Kc (RT )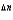 .
.
E)KpKc (RT )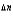 .
.
A)Kp Kc.
B)Kp Kc.
C)Kp Kc.
D)Kp Kc (RT )
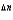 .
.E)KpKc (RT )
 .
.
Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
Under what conditions are the values of Kc and Kp for a given gas-phase equilibrium the same?
A)if there is no change in the moles of gas in the reaction
B)if there is no change in the temperature during the reaction
C)if the coefficients of the reactants and products are the same
D)if the pressure remains constant
E)if either Kc or Kp 1
A)if there is no change in the moles of gas in the reaction
B)if there is no change in the temperature during the reaction
C)if the coefficients of the reactants and products are the same
D)if the pressure remains constant
E)if either Kc or Kp 1

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
Two students measured an equilibrium constant for the same chemical reaction. Ken obtained a value of 130 for the equilibrium constant, but Barbie obtained a value of 11.4. The instructor checked their results and said they were both correct. How can that be?
A)The values vary according to the way the measurement is made.Ken must have measured product concentrations, while Barbie measured reactant concentrations.
B)The values vary according to the starting conditions of the reaction prior to equilibrium.Ken must have started with all reactants, while Barbie must have started with all products.
C)The values vary according to the stoichiometric coefficients that are used.The balancing coefficients that Ken used must have been twice those that Barbie used.
D)The values vary according to direction of the reaction.Ken must have used the reverse reaction.
E)The instructor must have made a mistake, because the equilibrium constant for a reaction must always be the same.
A)The values vary according to the way the measurement is made.Ken must have measured product concentrations, while Barbie measured reactant concentrations.
B)The values vary according to the starting conditions of the reaction prior to equilibrium.Ken must have started with all reactants, while Barbie must have started with all products.
C)The values vary according to the stoichiometric coefficients that are used.The balancing coefficients that Ken used must have been twice those that Barbie used.
D)The values vary according to direction of the reaction.Ken must have used the reverse reaction.
E)The instructor must have made a mistake, because the equilibrium constant for a reaction must always be the same.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine is 45.0 at a certain temperature. H2(g) I2(s)  2 HI(g)
2 HI(g)
Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are essentially the same.
 2 HI(g)
2 HI(g)Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are essentially the same.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
For the following reaction, Kc = 0.500 at 300K. What is the value of Kp? 2A(g) B(g)  2C(g) D(g)
2C(g) D(g)
A)8.26104
B)2.03102
C)0.500
D)12.3
E)303
 2C(g) D(g)
2C(g) D(g)A)8.26104
B)2.03102
C)0.500
D)12.3
E)303

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
Water can decompose at an elevated temperature to give hydrogen and oxygen according to the equation below. At a particular temperature, the partial pressures of H2O, H2, and O2 are 0.055 atm, 0.0065 atm, and 0.0045 atm, respectively, at equilibrium. What is the value of the equilibrium constant, KP, for this reaction at this temperature? 2H2O(g) 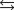 2H2(g) O2(g)
2H2(g) O2(g)
A)5.3 104
B)3.5 106
C)6.3 105
D)1.6 104
E)3.0 104
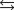 2H2(g) O2(g)
2H2(g) O2(g)A)5.3 104
B)3.5 106
C)6.3 105
D)1.6 104
E)3.0 104

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
Sulfur dioxide, gaseous water, and oxygen gas react to prepare sulfuric acid in the following unbalanced equation. 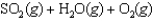
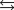
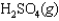 Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.
Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.
What is the value of the equilibrium constant, Kp, for the reaction?
A)3.30 104
B)5.00104
C)2.20103
D)3.03103
E)1.21106
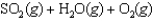
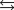
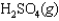 Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.
Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.What is the value of the equilibrium constant, Kp, for the reaction?
A)3.30 104
B)5.00104
C)2.20103
D)3.03103
E)1.21106

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
For the following hypothetical equilibrium, what is the value of the equilibrium constant if the concentrations at equilibrium are as shown? A 2 B ![<strong>For the following hypothetical equilibrium, what is the value of the equilibrium constant if the concentrations at equilibrium are as shown? <sub> </sub>A <font face=symbol></font> 2<sub> </sub>B C [A] <font face=symbol></font> 4.5 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>5 </sup>M [B] <font face=symbol></font> 2.2 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>2 </sup>M [C] <font face=symbol></font> 9.4 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>3 </sup>M</strong> A)0.22 B)9.9 C)4.3 <font face=symbol></font> 10<sup>5</sup> D)2.3 <font face=symbol></font> 10<sup>8</sup> E)9.5 <font face=symbol></font> 10<sup>3</sup>](https://storage.examlex.com/TB3835/11eaa952_9b52_5d62_ae90_272c9dc83841_TB3835_11.jpg) C
C
[A] 4.5 105 M
[B] 2.2 102 M
[C] 9.4 103 M
A)0.22
B)9.9
C)4.3 105
D)2.3 108
E)9.5 103
![<strong>For the following hypothetical equilibrium, what is the value of the equilibrium constant if the concentrations at equilibrium are as shown? <sub> </sub>A <font face=symbol></font> 2<sub> </sub>B C [A] <font face=symbol></font> 4.5 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>5 </sup>M [B] <font face=symbol></font> 2.2 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>2 </sup>M [C] <font face=symbol></font> 9.4 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>3 </sup>M</strong> A)0.22 B)9.9 C)4.3 <font face=symbol></font> 10<sup>5</sup> D)2.3 <font face=symbol></font> 10<sup>8</sup> E)9.5 <font face=symbol></font> 10<sup>3</sup>](https://storage.examlex.com/TB3835/11eaa952_9b52_5d62_ae90_272c9dc83841_TB3835_11.jpg) C
C[A] 4.5 105 M
[B] 2.2 102 M
[C] 9.4 103 M
A)0.22
B)9.9
C)4.3 105
D)2.3 108
E)9.5 103

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
Equilibrium constants can be expressed in terms of molar concentration, Kc, or partial pressure, KP. To relate these two equilibrium constants to each other, ________
A)each concentration must be multiplied by the factor RT.
B)each pressure must be multiplied by the factor RT.
C)Kc must be multiplied by the factor RT.
D)KP must be multiplied by the factor RT.
E)nothing must be done because their values are equal.
A)each concentration must be multiplied by the factor RT.
B)each pressure must be multiplied by the factor RT.
C)Kc must be multiplied by the factor RT.
D)KP must be multiplied by the factor RT.
E)nothing must be done because their values are equal.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
Coal (C) can be heated with steam (H2O) at high temperatures to produce a mixture of hydrogen and carbon monoxide gases. Methanol, which is a useful fuel, can be produced from these gases by the reaction below. At a particular high temperature, the equilibrium concentrations are the following: [H2] 0.095 mol/L, [CO] 0.035 mol/L, and [CH3OH] 0.065 mol/L. What is the value of the equilibrium constant, Kc, for this reaction at this temperature?
2H2(g) CO(g)![<strong>Coal (C) can be heated with steam (H<sub>2</sub>O) at high temperatures to produce a mixture of hydrogen and carbon monoxide gases. Methanol, which is a useful fuel, can be produced from these gases by the reaction below. At a particular high temperature, the equilibrium concentrations are the following: [H<sub>2</sub>] <font face=symbol></font> 0.095 mol/L, [CO] <font face=symbol></font> 0.035 mol/L, and [CH<sub>3</sub>OH] <font face=symbol></font> 0.065 mol/L. What is the value of the equilibrium constant, K<sub>c</sub>, for this reaction at this temperature? 2H<sub>2</sub>(g) <font face=symbol></font> CO(g) CH<sub>3</sub>OH(g)</strong> A)4.70 B)290 C)206 D)19.5 E)4.86](https://storage.examlex.com/TB3835/11eaa952_9b52_f9a7_ae90_1b4e6e29cc2b_TB3835_11.jpg) CH3OH(g)
CH3OH(g)
A)4.70
B)290
C)206
D)19.5
E)4.86
2H2(g) CO(g)
![<strong>Coal (C) can be heated with steam (H<sub>2</sub>O) at high temperatures to produce a mixture of hydrogen and carbon monoxide gases. Methanol, which is a useful fuel, can be produced from these gases by the reaction below. At a particular high temperature, the equilibrium concentrations are the following: [H<sub>2</sub>] <font face=symbol></font> 0.095 mol/L, [CO] <font face=symbol></font> 0.035 mol/L, and [CH<sub>3</sub>OH] <font face=symbol></font> 0.065 mol/L. What is the value of the equilibrium constant, K<sub>c</sub>, for this reaction at this temperature? 2H<sub>2</sub>(g) <font face=symbol></font> CO(g) CH<sub>3</sub>OH(g)</strong> A)4.70 B)290 C)206 D)19.5 E)4.86](https://storage.examlex.com/TB3835/11eaa952_9b52_f9a7_ae90_1b4e6e29cc2b_TB3835_11.jpg) CH3OH(g)
CH3OH(g)A)4.70
B)290
C)206
D)19.5
E)4.86

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate K for the following reaction, provided the concentration versus time graph shown below. 3A 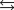 B 2C
B 2C 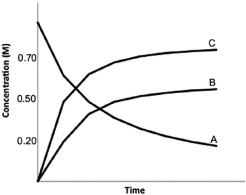
A)2.0
B)10
C)22
D)31
E)44
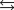 B 2C
B 2C 
A)2.0
B)10
C)22
D)31
E)44

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
Equilibrium constants for gases can be expressed in terms of concentrations, Kc, or in terms of partial pressures, Kp. Which one of the following statements regarding Kc and Kp is correct?
A)Kc and Kp are equal when all stoichiometric coefficients in the balanced reaction equation equal one.
B)Kc and Kp have the same values but different units.
C)Kc and Kp are equal when the sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)Kc and Kp can never be equal.
E)Kc and Kp are equal when the conditions are standard (P 1 atm, T 298 K).
A)Kc and Kp are equal when all stoichiometric coefficients in the balanced reaction equation equal one.
B)Kc and Kp have the same values but different units.
C)Kc and Kp are equal when the sum of the stoichiometric coefficients for the products equals the sum of the stoichiometric coefficients for the reactants.
D)Kc and Kp can never be equal.
E)Kc and Kp are equal when the conditions are standard (P 1 atm, T 298 K).

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
Which expression corresponds to the equilibrium constant Kc for the reaction written as 2NH3(g) 2O2(g) ![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11a_ae90_79378eef2f01_TB3835_11.jpg) N2O(g) 3H2O2(g)
N2O(g) 3H2O2(g)
A)![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11b_ae90_39dbd48dec8e_TB3835_11.jpg)
B)![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11c_ae90_f3473593860a_TB3835_11.jpg)
C)![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_e82d_ae90_bb7433503da7_TB3835_11.jpg)
D)[N2O][H2O2]3
E)![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_e82e_ae90_db5e67ea0939_TB3835_11.jpg)
![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11a_ae90_79378eef2f01_TB3835_11.jpg) N2O(g) 3H2O2(g)
N2O(g) 3H2O2(g)A)
![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11b_ae90_39dbd48dec8e_TB3835_11.jpg)
B)
![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_c11c_ae90_f3473593860a_TB3835_11.jpg)
C)
![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_e82d_ae90_bb7433503da7_TB3835_11.jpg)
D)[N2O][H2O2]3
E)
![<strong>Which expression corresponds to the equilibrium constant K<sub>c</sub> for the reaction written as 2NH<sub>3</sub>(g) <font face=symbol></font> 2O<sub>2</sub>(g) N<sub>2</sub>O(g) <font face=symbol></font> 3H<sub>2</sub>O<sub>2</sub>(g)</strong> A) B) C) D)[N<sub>2</sub>O][H<sub>2</sub>O<sub>2</sub>]<sup>3</sup> E)](https://storage.examlex.com/TB3835/11eaa952_9b51_e82e_ae90_db5e67ea0939_TB3835_11.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
The chemical equilibrium constant for the following reaction is 2.15 102. 2NO2(g)  N2O4(g)
N2O4(g)
What is the value of the equilibrium constant for the following reaction?
6NO2(g) 3N2O4(g)
3N2O4(g)
A)6.45 102
B)9.94 106
C)1.01 107
D)1.55 103
E)4.65 102
 N2O4(g)
N2O4(g)What is the value of the equilibrium constant for the following reaction?
6NO2(g)
 3N2O4(g)
3N2O4(g)A)6.45 102
B)9.94 106
C)1.01 107
D)1.55 103
E)4.65 102

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
An increase in the number of moles of gas as the reaction goes from reactants to products in a gas-phase equilibrium results in ________
A)Kp Kc.
B)Kp Kc.
C)Kp Kc.
D)Kp Kc (RT ) .
.
E)KpKc (RT ) .
.
A)Kp Kc.
B)Kp Kc.
C)Kp Kc.
D)Kp Kc (RT )
 .
.E)KpKc (RT )
 .
.
Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
The equilibrium constant for the acid ionization of mercaptoethanol (HSCH2CH2OH ) is 1.91 1010. HSCH2CH2OH(aq) 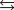 H(aq) SCH2CH2OH(aq)
H(aq) SCH2CH2OH(aq)
Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies far to the right.
IV.Equilibrium lies far to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are comparable.
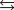 H(aq) SCH2CH2OH(aq)
H(aq) SCH2CH2OH(aq)Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies far to the right.
IV.Equilibrium lies far to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are comparable.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
The equilibrium constant for the formation of calcium carbonate from the ions in solution is 2.2 108 according to the reaction: 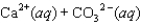
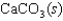 What is the value of the equilibrium constant for the reverse of this reaction?
What is the value of the equilibrium constant for the reverse of this reaction?
A)the same, 2.2 108
B)(2.2 108)
C)2.2 108
D)4.5 109
E)4.5 109
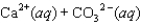
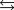
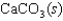 What is the value of the equilibrium constant for the reverse of this reaction?
What is the value of the equilibrium constant for the reverse of this reaction?A)the same, 2.2 108
B)(2.2 108)
C)2.2 108
D)4.5 109
E)4.5 109

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
For the following reaction, Kp = 137 at 3.50 102K. What is the value of Kc? 2C4H10(l) 3O2(g)  8CO2(g) 10H2O(g)
8CO2(g) 10H2O(g)
A)7.03106
B)5.79103
C)4.77
D)3.24106
E)2.67109
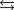 8CO2(g) 10H2O(g)
8CO2(g) 10H2O(g)A)7.03106
B)5.79103
C)4.77
D)3.24106
E)2.67109

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
In the following reaction, which of the statements is true, given the concentrations of each species? N2(g) 3 H2(g) ![<strong>In the following reaction, which of the statements is true, given the concentrations of each species? N<sub>2</sub>(g) <font face=symbol></font> 3 H<sub>2</sub>(g) 2 NH<sub>3</sub>(g) K <font face=symbol></font> 9.60 [N<sub>2</sub>] <font face=symbol></font> [H<sub>2</sub>] <font face=symbol></font> 0.100 M and [NH<sub>3</sub>] <font face=symbol></font> 0.352 M</strong> A)The reaction is at equilibrium. B)More ammonia must form to achieve equilibrium. C)More nitrogen and hydrogen must form to achieve equilibrium. D)More nitrogen must be consumed to achieve equilibrium. E)Equilibrium cannot be established in the system.](https://storage.examlex.com/TB3835/11eaa952_9b59_627e_ae90_91df19d2f358_TB3835_11.jpg) 2 NH3(g) K 9.60
2 NH3(g) K 9.60
[N2] [H2] 0.100 M and [NH3] 0.352 M
A)The reaction is at equilibrium.
B)More ammonia must form to achieve equilibrium.
C)More nitrogen and hydrogen must form to achieve equilibrium.
D)More nitrogen must be consumed to achieve equilibrium.
E)Equilibrium cannot be established in the system.
![<strong>In the following reaction, which of the statements is true, given the concentrations of each species? N<sub>2</sub>(g) <font face=symbol></font> 3 H<sub>2</sub>(g) 2 NH<sub>3</sub>(g) K <font face=symbol></font> 9.60 [N<sub>2</sub>] <font face=symbol></font> [H<sub>2</sub>] <font face=symbol></font> 0.100 M and [NH<sub>3</sub>] <font face=symbol></font> 0.352 M</strong> A)The reaction is at equilibrium. B)More ammonia must form to achieve equilibrium. C)More nitrogen and hydrogen must form to achieve equilibrium. D)More nitrogen must be consumed to achieve equilibrium. E)Equilibrium cannot be established in the system.](https://storage.examlex.com/TB3835/11eaa952_9b59_627e_ae90_91df19d2f358_TB3835_11.jpg) 2 NH3(g) K 9.60
2 NH3(g) K 9.60[N2] [H2] 0.100 M and [NH3] 0.352 M
A)The reaction is at equilibrium.
B)More ammonia must form to achieve equilibrium.
C)More nitrogen and hydrogen must form to achieve equilibrium.
D)More nitrogen must be consumed to achieve equilibrium.
E)Equilibrium cannot be established in the system.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
Given the following reactions and associated equilibrium constants 
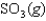 K1 3.1
K1 3.1 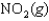
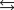
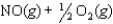 K2 4.5 what is the equilibrium constant for the following reaction?
K2 4.5 what is the equilibrium constant for the following reaction?
SO3(g) NO(g)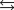 SO2(g) NO2(g) Knew ?
SO2(g) NO2(g) Knew ?
A)1.45
B)7.17 10-2
C)14.0
D)6.89 10-1
E)163
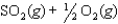
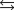
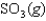 K1 3.1
K1 3.1 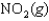
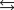
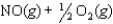 K2 4.5 what is the equilibrium constant for the following reaction?
K2 4.5 what is the equilibrium constant for the following reaction?SO3(g) NO(g)
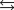 SO2(g) NO2(g) Knew ?
SO2(g) NO2(g) Knew ?A)1.45
B)7.17 10-2
C)14.0
D)6.89 10-1
E)163

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
If the reaction quotient Q has a smaller value than the related equilibrium constant, K, ________
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will decrease until it is equal to Q.
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will decrease until it is equal to Q.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
The equilibrium constants for the two reactions are known: Mm 4L ![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_7c32_ae90_ef8772aaaf40_TB3835_11.jpg) [ML4]m4 Ka
[ML4]m4 Ka
HL![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_a343_ae90_bf4fb69a3e93_TB3835_11.jpg) H L Kb
H L Kb
What must be the value of the equilibrium constant, Koverall for the following overall reaction?
Mm 4HL![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_a344_ae90_5f7b6a598591_TB3835_11.jpg) [ML4]m4 4H Koverall
[ML4]m4 4H Koverall
A)KaKb
B)Ka 4Kb
C)Ka Kb4
D)KaKb4
E)KaKb1/4
![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_7c32_ae90_ef8772aaaf40_TB3835_11.jpg) [ML4]m4 Ka
[ML4]m4 KaHL
![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_a343_ae90_bf4fb69a3e93_TB3835_11.jpg) H L Kb
H L KbWhat must be the value of the equilibrium constant, Koverall for the following overall reaction?
Mm 4HL
![<strong>The equilibrium constants for the two reactions are known: M<sup>m</sup><font face=symbol><sup></sup></font><font face=symbol></font> 4L<font face=symbol><sup></sup></font> [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> K<sub>a</sub> HL H<font face=symbol><sup></sup></font><font face=symbol></font> L<font face=symbol><sup></sup></font> K<sub>b</sub> What must be the value of the equilibrium constant, K<sub>overall</sub> for the following overall reaction? M<sup>m</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4HL [ML<sub>4</sub>]<sup>m</sup><font face=symbol><sup></sup></font><sup>4</sup> <font face=symbol></font> 4H<font face=symbol><sup></sup></font> K<sub>overall</sub></strong> A)K<sub>a</sub>K<sub>b</sub> B)K<sub>a</sub> <font face=symbol></font> 4K<sub>b</sub> C)K<sub>a</sub> <font face=symbol></font> K<sub>b</sub><sup>4</sup> D)K<sub>a</sub>K<sub>b</sub><sup>4</sup> E)K<sub>a</sub>K<sub>b</sub><sup>1/4</sup>](https://storage.examlex.com/TB3835/11eaa952_9b56_a344_ae90_5f7b6a598591_TB3835_11.jpg) [ML4]m4 4H Koverall
[ML4]m4 4H KoverallA)KaKb
B)Ka 4Kb
C)Ka Kb4
D)KaKb4
E)KaKb1/4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
The chemical equilibrium constant for the following reaction is 14.5. CH3OH(g) 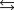 CO(g) 2H2(g)
CO(g) 2H2(g)
What is the value of the equilibrium constant for the following reaction?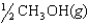
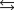
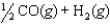
A)3.81
B)2.62 101
C)7.25
D)1.38 101
E)6.90 102
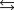 CO(g) 2H2(g)
CO(g) 2H2(g)What is the value of the equilibrium constant for the following reaction?
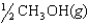
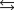
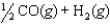
A)3.81
B)2.62 101
C)7.25
D)1.38 101
E)6.90 102

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) SO2(g) ![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_d6b0_ae90_9120e52b436d_TB3835_00.jpg) CaSO3(s)
CaSO3(s)
A)Kc [SO2]
B)Kc ![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_d6b1_ae90_95fbee4c33c7_TB3835_11.jpg)
C)Kc [CaO] [SO2]
D)Kc ![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_fdc2_ae90_c768e7c4390f_TB3835_11.jpg)
E)Kc [CaSO3] [CaO] [SO2]
![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_d6b0_ae90_9120e52b436d_TB3835_00.jpg) CaSO3(s)
CaSO3(s)A)Kc [SO2]
B)Kc
![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_d6b1_ae90_95fbee4c33c7_TB3835_11.jpg)
C)Kc [CaO] [SO2]
D)Kc
![<strong>Which of the following is the equilibrium expression for the scrubbing reaction of calcium oxide and sulfur dioxide to remove sulfur dioxide from the exhaust gases of coal-fired power plants? CaO(s) <font face=symbol></font> SO<sub>2</sub>(g) CaSO<sub>3</sub>(s)</strong> A)K<sub>c</sub> <font face=symbol></font> [SO<sub>2</sub>] B)K<sub>c</sub> <font face=symbol></font> C)K<sub>c</sub> <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>] D)K<sub>c</sub> <font face=symbol></font> E)K<sub>c</sub> <font face=symbol></font> [CaSO<sub>3</sub>] <font face=symbol></font> [CaO] <font face=symbol></font> [SO<sub>2</sub>]](https://storage.examlex.com/TB3835/11eaa952_9b59_fdc2_ae90_c768e7c4390f_TB3835_11.jpg)
E)Kc [CaSO3] [CaO] [SO2]

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
Given the following reactions and associated equilibrium constants 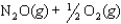
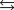 2NO(g) K1 1.70 1013
2NO(g) K1 1.70 1013 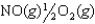
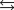 NO2(g)
NO2(g)
K2 6.83 106
What is the equilibrium constant for the following reaction?
2N2O(g) 3O2(g)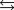 4NO2(g) Knew ?
4NO2(g) Knew ?
A)1.59 102
B)9.29 1018
C)40.3
D)62.9
E)1.08 1017
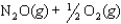
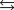 2NO(g) K1 1.70 1013
2NO(g) K1 1.70 1013 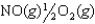
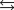 NO2(g)
NO2(g)K2 6.83 106
What is the equilibrium constant for the following reaction?
2N2O(g) 3O2(g)
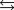 4NO2(g) Knew ?
4NO2(g) Knew ?A)1.59 102
B)9.29 1018
C)40.3
D)62.9
E)1.08 1017

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
Sulfur dioxide emitted in the burning of coal is "scrubbed" from the effluent using ________
A)limestone (CaCO3).
B)lime (CaO).
C)calcium metal (Ca).
D)calcium hydride (CaH2).
E)calcium hydroxide [Ca(OH)2].
A)limestone (CaCO3).
B)lime (CaO).
C)calcium metal (Ca).
D)calcium hydride (CaH2).
E)calcium hydroxide [Ca(OH)2].

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
Write the expression for the reaction quotient for the reaction Cu2(aq) 4NH3(aq) ![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_7817_ae90_d561dbb43bef_TB3835_11.jpg) Cu(NH3)42(aq)
Cu(NH3)42(aq)
A)Q = [Cu2] 4[NH3] [Cu(NH3)42]
B)![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_7818_ae90_313dfaaa25c5_TB3835_11.jpg)
C)![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_9f29_ae90_e1c84558766b_TB3835_11.jpg)
D)![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_9f2a_ae90_2958f4b285b7_TB3835_11.jpg)
E)![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_c63b_ae90_4f391e24de60_TB3835_11.jpg)
![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_7817_ae90_d561dbb43bef_TB3835_11.jpg) Cu(NH3)42(aq)
Cu(NH3)42(aq)A)Q = [Cu2] 4[NH3] [Cu(NH3)42]
B)
![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_7818_ae90_313dfaaa25c5_TB3835_11.jpg)
C)
![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_9f29_ae90_e1c84558766b_TB3835_11.jpg)
D)
![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_9f2a_ae90_2958f4b285b7_TB3835_11.jpg)
E)
![<strong>Write the expression for the reaction quotient for the reaction Cu<sup>2</sup><font face=symbol><sup></sup></font>(aq) <font face=symbol></font> 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>(aq)</strong> A)Q = [Cu<sup>2</sup><font face=symbol><sup></sup></font>] <font face=symbol></font> 4[NH<sub>3</sub>] <font face=symbol></font> [Cu(NH<sub>3</sub>)<sub>4</sub><sup>2</sup><font face=symbol><sup></sup></font>] B) C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b58_c63b_ae90_4f391e24de60_TB3835_11.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
Which statement about an equilibrium constant, K, and a reaction quotient, Q, for a reaction is correct?
A)Values for each can be determined using units of moles/liter or atmospheres.
B)K is the reciprocal of Q: K 1/Q.
C)K always is larger than Q.
D)Q always is larger than K.
E)Q can never equal K.
A)Values for each can be determined using units of moles/liter or atmospheres.
B)K is the reciprocal of Q: K 1/Q.
C)K always is larger than Q.
D)Q always is larger than K.
E)Q can never equal K.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
If the reaction quotient Q is equal to the related equilibrium constant, K, ________
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
The equilibrium constant for the reaction below is 1.3102. 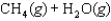
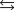
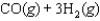 What is the value of the equilibrium constant for the following reaction?
What is the value of the equilibrium constant for the following reaction? 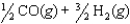
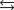
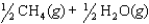
A)1.69 104
B)1.30 102
C)1.14 101
D)8.77
E)76.9
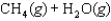
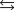
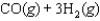 What is the value of the equilibrium constant for the following reaction?
What is the value of the equilibrium constant for the following reaction? 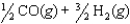
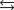
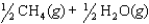
A)1.69 104
B)1.30 102
C)1.14 101
D)8.77
E)76.9

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
A series of four equilibrium steps can be defined for the following overall reaction: Cu2 4NH3 ![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f165_ae90_51617c053246_TB3835_11.jpg) [Cu(NH3)4]2Koverall
[Cu(NH3)4]2Koverall
Cu2 NH3![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f166_ae90_bb231e6dd13e_TB3835_11.jpg) [Cu(NH3)]2K1
[Cu(NH3)]2K1
[Cu(NH3)]2 NH3![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f167_ae90_0df659dabb69_TB3835_11.jpg) [Cu(NH3)2]2K2
[Cu(NH3)2]2K2
Etc)
What is true about K4?
A)K4 Koverall
B)K4 K1K2K3
C)![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_1878_ae90_75e780f4e2b6_TB3835_11.jpg)
D)![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_1879_ae90_cbefb4584b08_TB3835_11.jpg)
E)![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_3f8a_ae90_477b8b45cb56_TB3835_11.jpg)
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f165_ae90_51617c053246_TB3835_11.jpg) [Cu(NH3)4]2Koverall
[Cu(NH3)4]2KoverallCu2 NH3
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f166_ae90_bb231e6dd13e_TB3835_11.jpg) [Cu(NH3)]2K1
[Cu(NH3)]2K1[Cu(NH3)]2 NH3
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b56_f167_ae90_0df659dabb69_TB3835_11.jpg) [Cu(NH3)2]2K2
[Cu(NH3)2]2K2Etc)
What is true about K4?
A)K4 Koverall
B)K4 K1K2K3
C)
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_1878_ae90_75e780f4e2b6_TB3835_11.jpg)
D)
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_1879_ae90_cbefb4584b08_TB3835_11.jpg)
E)
![<strong>A series of four equilibrium steps can be defined for the following overall reaction: Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> 4NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>4</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>overall</sub> Cu<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>1</sub> [Cu(NH<sub>3</sub>)]<sup>2</sup><font face=symbol><sup></sup></font> <font face=symbol></font> NH<sub>3</sub> [Cu(NH<sub>3</sub>)<sub>2</sub>]<sup>2</sup><font face=symbol><sup></sup></font>K<sub>2</sub> Etc) What is true about K<sub>4</sub>?</strong> A)K<sub>4</sub> <font face=symbol></font> K<sub>overall</sub> B)K<sub>4</sub> <font face=symbol></font> K<sub>1</sub>K<sub>2</sub>K<sub>3</sub> C) D) E)](https://storage.examlex.com/TB3835/11eaa952_9b57_3f8a_ae90_477b8b45cb56_TB3835_11.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
In equilibrium expressions, the concentrations of pure solids and liquids ________
A)have the assigned value of one.
B)have the assigned value of zero.
C)have constant values, cS and cl.
D)are determined from the density and molar mass.
E)are treated as any other solute.
A)have the assigned value of one.
B)have the assigned value of zero.
C)have constant values, cS and cl.
D)are determined from the density and molar mass.
E)are treated as any other solute.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following is the equilibrium expression, KP, for the reaction that produces lime, CaO, from limestone, CaCO3? CaCO3(s) ![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_24d3_ae90_a91790d9bb02_TB3835_11.jpg) CaO(s) CO2(g)
CaO(s) CO2(g)
A)![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_24d4_ae90_c15f57ad6750_TB3835_11.jpg)
B)![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_4be5_ae90_cbee6dd55aad_TB3835_11.jpg)
C)![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_4be6_ae90_0d6aaebe9b09_TB3835_11.jpg)
D)[CaO][CO2]
E)![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_72f7_ae90_c5fa8da418a6_TB3835_11.jpg)
![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_24d3_ae90_a91790d9bb02_TB3835_11.jpg) CaO(s) CO2(g)
CaO(s) CO2(g)A)
![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_24d4_ae90_c15f57ad6750_TB3835_11.jpg)
B)
![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_4be5_ae90_cbee6dd55aad_TB3835_11.jpg)
C)
![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_4be6_ae90_0d6aaebe9b09_TB3835_11.jpg)
D)[CaO][CO2]
E)
![<strong>Which one of the following is the equilibrium expression, K<sub>P</sub>, for the reaction that produces lime, CaO, from limestone, CaCO<sub>3</sub>? CaCO<sub>3</sub>(s) CaO(s) <font face=symbol></font> CO<sub>2</sub>(g)</strong> A) B) C) D)[CaO][CO<sub>2</sub>] E)](https://storage.examlex.com/TB3835/11eaa952_9b5a_72f7_ae90_c5fa8da418a6_TB3835_11.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
The equilibrium constant for the reaction below is 17.5. S(s) 3 O2(g)  2 SO3(g)
2 SO3(g)
What is the value of the equilibrium constant for the following reaction?
6 SO3(g) 3S(s) 9 O2(g)
3S(s) 9 O2(g)
A)1.87 104
B)5.71 102
C)17.5
D)306
E)5360
 2 SO3(g)
2 SO3(g)What is the value of the equilibrium constant for the following reaction?
6 SO3(g)
 3S(s) 9 O2(g)
3S(s) 9 O2(g)A)1.87 104
B)5.71 102
C)17.5
D)306
E)5360

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
In the following reaction, which of the statements is true, given the concentrations of each species? C6H6(g) 3H2(g) ![<strong>In the following reaction, which of the statements is true, given the concentrations of each species? C<sub>6</sub>H<sub>6</sub>(g) <font face=symbol></font> 3H<sub>2</sub>(g) C<sub>6</sub>H<sub>12</sub>(g) K <font face=symbol></font> 1.23<font face=symbol></font><font face=symbol></font>10<font face=symbol><sup></sup></font><sup>3</sup> [C<sub>6</sub>H<sub>6</sub>] <font face=symbol></font> [H<sub>2</sub>] <font face=symbol></font> 0.170 M and [C<sub>6</sub>H<sub>12</sub>] <font face=symbol></font> 0.025 M</strong> A)More hydrogen must be consumed to achieve equilibrium. B)More cyclohexane (C<sub>6</sub>H<sub>12</sub>) must form to achieve equilibrium. C)The reaction is at equilibrium. D)More benzene (C<sub>6</sub>H<sub>6</sub>) must be formed to achieve equilibrium. E)Equilibrium cannot be established in the system.](https://storage.examlex.com/TB3835/11eaa952_9b59_898f_ae90_896d6cf0941b_TB3835_00.jpg) C6H12(g) K 1.23103
C6H12(g) K 1.23103
[C6H6] [H2] 0.170 M and [C6H12] 0.025 M
A)More hydrogen must be consumed to achieve equilibrium.
B)More cyclohexane (C6H12) must form to achieve equilibrium.
C)The reaction is at equilibrium.
D)More benzene (C6H6) must be formed to achieve equilibrium.
E)Equilibrium cannot be established in the system.
![<strong>In the following reaction, which of the statements is true, given the concentrations of each species? C<sub>6</sub>H<sub>6</sub>(g) <font face=symbol></font> 3H<sub>2</sub>(g) C<sub>6</sub>H<sub>12</sub>(g) K <font face=symbol></font> 1.23<font face=symbol></font><font face=symbol></font>10<font face=symbol><sup></sup></font><sup>3</sup> [C<sub>6</sub>H<sub>6</sub>] <font face=symbol></font> [H<sub>2</sub>] <font face=symbol></font> 0.170 M and [C<sub>6</sub>H<sub>12</sub>] <font face=symbol></font> 0.025 M</strong> A)More hydrogen must be consumed to achieve equilibrium. B)More cyclohexane (C<sub>6</sub>H<sub>12</sub>) must form to achieve equilibrium. C)The reaction is at equilibrium. D)More benzene (C<sub>6</sub>H<sub>6</sub>) must be formed to achieve equilibrium. E)Equilibrium cannot be established in the system.](https://storage.examlex.com/TB3835/11eaa952_9b59_898f_ae90_896d6cf0941b_TB3835_00.jpg) C6H12(g) K 1.23103
C6H12(g) K 1.23103[C6H6] [H2] 0.170 M and [C6H12] 0.025 M
A)More hydrogen must be consumed to achieve equilibrium.
B)More cyclohexane (C6H12) must form to achieve equilibrium.
C)The reaction is at equilibrium.
D)More benzene (C6H6) must be formed to achieve equilibrium.
E)Equilibrium cannot be established in the system.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
Gaseous hydrogen and iodine can be used to prepare HI in the following balanced chemical reaction. Calculate a value for the reaction quotient when [H2] [I2] 4.67 10-3 M and [HI] 4.07 10-2 M. H2(g) I2(g) ![<strong>Gaseous hydrogen and iodine can be used to prepare HI in the following balanced chemical reaction. Calculate a value for the reaction quotient when [H<sub>2</sub>] <font face=symbol></font> [I<sub>2</sub>] <font face=symbol></font> 4.67 <font face=symbol></font>10<sup>-</sup><sup>3</sup> M and [HI] <font face=symbol></font> 4.07 <font face=symbol></font> 10<sup>-</sup><sup>2 </sup>M. H<sub>2</sub>(g) <font face=symbol></font> I<sub>2</sub>(g) 2HI(g)</strong> A)1.32 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>2</sup> B)1.87 <font face=symbol></font> 10<sup>3</sup> C)76.0 D)5.36 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>4</sup> E)1.77 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>1</sup>](https://storage.examlex.com/TB3835/11eaa952_9b58_c63c_ae90_b181fa215aec_TB3835_11.jpg) 2HI(g)
2HI(g)
A)1.32 102
B)1.87 103
C)76.0
D)5.36 104
E)1.77 101
![<strong>Gaseous hydrogen and iodine can be used to prepare HI in the following balanced chemical reaction. Calculate a value for the reaction quotient when [H<sub>2</sub>] <font face=symbol></font> [I<sub>2</sub>] <font face=symbol></font> 4.67 <font face=symbol></font>10<sup>-</sup><sup>3</sup> M and [HI] <font face=symbol></font> 4.07 <font face=symbol></font> 10<sup>-</sup><sup>2 </sup>M. H<sub>2</sub>(g) <font face=symbol></font> I<sub>2</sub>(g) 2HI(g)</strong> A)1.32 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>2</sup> B)1.87 <font face=symbol></font> 10<sup>3</sup> C)76.0 D)5.36 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>4</sup> E)1.77 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>1</sup>](https://storage.examlex.com/TB3835/11eaa952_9b58_c63c_ae90_b181fa215aec_TB3835_11.jpg) 2HI(g)
2HI(g)A)1.32 102
B)1.87 103
C)76.0
D)5.36 104
E)1.77 101

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
Water can decompose at an elevated temperature to give hydrogen and oxygen according to the equation below. At a particular temperature in a vessel containing water vapor and air, the partial pressures of H2O, H2, and O2 are 0.055 atm, 0.0065 atm, and 0.19 atm, respectively. What is the value of the reaction quotient, QP, for this reaction under these conditions? 2H2O(g)  2H2(g) O2(g)
2H2(g) O2(g)
A)0.023
B)1.5 104
C)2.7 103
D)370
E)4.4 104
 2H2(g) O2(g)
2H2(g) O2(g)A)0.023
B)1.5 104
C)2.7 103
D)370
E)4.4 104

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
If the reaction quotient Q has a larger value than the related equilibrium constant, K, ________
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following reactions will not shift toward the reactants products when the pressure of the system is altered by changing the volume?
A)2CO2(g) 2 CO(g) O2(g)
2 CO(g) O2(g)
B)CaCO3(s) CaO(s) CO2(g)
CaO(s) CO2(g)
C)N2(g) 3H2(g) 2NH3(g)
2NH3(g)
D)H2(g) I2(g) 2HI(g)
2HI(g)
E)2SO2(g) O2(g) 2SO3(g)
2SO3(g)
A)2CO2(g)
 2 CO(g) O2(g)
2 CO(g) O2(g)B)CaCO3(s)
 CaO(s) CO2(g)
CaO(s) CO2(g)C)N2(g) 3H2(g)
 2NH3(g)
2NH3(g)D)H2(g) I2(g)
 2HI(g)
2HI(g)E)2SO2(g) O2(g)
 2SO3(g)
2SO3(g)
Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
In a simple equilibrium A B  C, will there be any stress to the system if both B and C are added to the equilibrium system simultaneously and in the same amount?
C, will there be any stress to the system if both B and C are added to the equilibrium system simultaneously and in the same amount?
A)No, these two stresses will always cancel each other out.
B)No, these two stresses will cancel each other out unless the initial concentrations of B and C are also the same.
C)Yes, the same amount of A is also required for the stresses to cancel each other out.
D)Yes, unless the initial concentrations of B and C are also the same.
E)More than two of the above statements are correct.
 C, will there be any stress to the system if both B and C are added to the equilibrium system simultaneously and in the same amount?
C, will there be any stress to the system if both B and C are added to the equilibrium system simultaneously and in the same amount?A)No, these two stresses will always cancel each other out.
B)No, these two stresses will cancel each other out unless the initial concentrations of B and C are also the same.
C)Yes, the same amount of A is also required for the stresses to cancel each other out.
D)Yes, unless the initial concentrations of B and C are also the same.
E)More than two of the above statements are correct.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
Cylinders of NO gas may contain small quantities of oxygen as an impurity, leading to the formation of NO2 in equilibrium with the NO and the oxygen. Is this contamination by NO2 dependent on the pressure in the tank?
A)Yes, there will be more NO2 at higher pressures.
B)Yes, there will be less NO2 at higher pressures.
C)No, the amount of NO2 has nothing to do with pressure.
D)No, the amount of NO2 depends on the partial pressure, not the total pressure.
E)There is no way to tell without additional information.
A)Yes, there will be more NO2 at higher pressures.
B)Yes, there will be less NO2 at higher pressures.
C)No, the amount of NO2 has nothing to do with pressure.
D)No, the amount of NO2 depends on the partial pressure, not the total pressure.
E)There is no way to tell without additional information.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following occurs when products are removed from a chemical reaction in solution or the gas phase at equilibrium?
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the removal of products.
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the removal of products.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
If the temperature of an endothermic reaction at equilibrium could be increased instantaneously, what would be the instantaneous effect on Q and K before equilibrium was again achieved?
A)Q would increase and K would stay the same.
B)Q would decrease and K would stay the same.
C)Q would stay the same and K would increase.
D)Q would stay the same and K would decrease.
E)Both Q and K would stay the same.
A)Q would increase and K would stay the same.
B)Q would decrease and K would stay the same.
C)Q would stay the same and K would increase.
D)Q would stay the same and K would decrease.
E)Both Q and K would stay the same.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Given the reaction below, which of the following would cause an increase in the concentration of products? 4NH3(g) 3O2(g)  2N2(g) 6H2O(g)
2N2(g) 6H2O(g)
I. addition of water
II. removal of ammonia
III. decrease of the volume of the container
IV. addition of oxygen
A)I only
B)II only
C)IV only
D)II and III
E)III and IV
 2N2(g) 6H2O(g)
2N2(g) 6H2O(g)I. addition of water
II. removal of ammonia
III. decrease of the volume of the container
IV. addition of oxygen
A)I only
B)II only
C)IV only
D)II and III
E)III and IV

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
Given the reaction below, which of the following would cause an increase in the concentration of reactants? 2HF(g)  F2(g) H2(g)
F2(g) H2(g)  H° 12.0 kJ/mol
H° 12.0 kJ/mol
I. removal of hydrogen
II. addition of HF
III. increasing the temperature of the reaction
IV. an increase in the volume of the container
A)I only
B)II only
C)III only
D)IV only
E)III and IV
 F2(g) H2(g)
F2(g) H2(g)  H° 12.0 kJ/mol
H° 12.0 kJ/molI. removal of hydrogen
II. addition of HF
III. increasing the temperature of the reaction
IV. an increase in the volume of the container
A)I only
B)II only
C)III only
D)IV only
E)III and IV

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
Carbon dioxide and argon, which is a noble or inert gas, are placed in a vessel and heated. Equilibrium is reached at some particular temperature. Carbon dioxide dissociates by the following reaction. 2CO2(g)  2CO(g) O2(g)
2CO(g) O2(g)
What is the result of increasing the pressure by adding more argon?
I. The equilibrium shifts to produce more carbon dioxide and reduce the pressure.
II. The equilibrium shifts to remove carbon dioxide and reduce the pressure.
III. The equilibrium does not change because argon is not involved in the reaction.
IV. The equilibrium does not change because the concentrations are not affected.
A)I only
B)II only
C)III only
D)IV only
E)III and IV
 2CO(g) O2(g)
2CO(g) O2(g)What is the result of increasing the pressure by adding more argon?
I. The equilibrium shifts to produce more carbon dioxide and reduce the pressure.
II. The equilibrium shifts to remove carbon dioxide and reduce the pressure.
III. The equilibrium does not change because argon is not involved in the reaction.
IV. The equilibrium does not change because the concentrations are not affected.
A)I only
B)II only
C)III only
D)IV only
E)III and IV

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
What happens to the equilibrium between NO2(g) and N2O4(g) in inert argon when the volume is increased and additional argon is added to maintain a constant total pressure?
A)The ratio of NO2 to N2O4 increases solely because of the increase in volume.
B)The ratio of NO2 to N2O4 increases solely because of the addition of argon.
C)The ratio of NO2 to N2O4 decreases solely because of the increase in volume.
D)The ratio of NO2 to N2O4 decreases solely because of the addition of argon.
E)The ratio of NO2 to N2O4 remains the same, because the effects of the two processes cancel each other out.
A)The ratio of NO2 to N2O4 increases solely because of the increase in volume.
B)The ratio of NO2 to N2O4 increases solely because of the addition of argon.
C)The ratio of NO2 to N2O4 decreases solely because of the increase in volume.
D)The ratio of NO2 to N2O4 decreases solely because of the addition of argon.
E)The ratio of NO2 to N2O4 remains the same, because the effects of the two processes cancel each other out.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
In the Reaction, Initial, Change, Equilibrium (RICE) table started for calculating equilibrium concentrations of the reaction shown, the terms in the "change" row are ________ 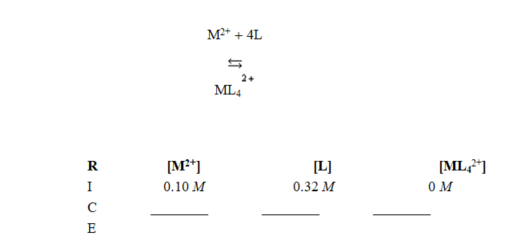
A)(x, x, x.)
B)(x, x, x.)
C)(x, 4x, x.)
D)(x, 4x, x.)
E)(x, 4x, x.)

A)(x, x, x.)
B)(x, x, x.)
C)(x, 4x, x.)
D)(x, 4x, x.)
E)(x, 4x, x.)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
Solid mercury(II) oxide decomposes when heated to produce liquid mercury and oxygen. 2HgO(s) 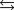 2Hg(l) O2(g)
2Hg(l) O2(g)
An amount of mercury(II) oxide is placed in a vessel at a particular temperature and allowed to reach equilibrium. How could the amount of liquid mercury in the vessel be increased?
I. adding more mercury(II) oxide
II. removing some oxygen
III. increasing the volume of the vessel
A)I only
B)II only
C)III only
D)I and II
E)II and III
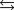 2Hg(l) O2(g)
2Hg(l) O2(g)An amount of mercury(II) oxide is placed in a vessel at a particular temperature and allowed to reach equilibrium. How could the amount of liquid mercury in the vessel be increased?
I. adding more mercury(II) oxide
II. removing some oxygen
III. increasing the volume of the vessel
A)I only
B)II only
C)III only
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is true regarding the effect of a catalyst on chemical equilibrium?
A)Only the forward rate increases, so the quantity of products increases.
B)Only the forward rate increases, but the quantity of products remains the same.
C)Both the forward and reverse rates increase, and the quantity of products increases.
D)Both the forward and reverse rates increase, but the quantity of products is unchanged.
E)The effect varies depending on whether the reaction is endothermic or exothermic.
A)Only the forward rate increases, so the quantity of products increases.
B)Only the forward rate increases, but the quantity of products remains the same.
C)Both the forward and reverse rates increase, and the quantity of products increases.
D)Both the forward and reverse rates increase, but the quantity of products is unchanged.
E)The effect varies depending on whether the reaction is endothermic or exothermic.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
For a chemical reaction at equilibrium, which of the following will change the value of the equilibrium constant K?
I.changing the temperature
II.changing the total concentration of reactants and products
III.changing the reaction coefficients
A)I only
B)II only
C)III only
D)I and II only
E)I and III only
I.changing the temperature
II.changing the total concentration of reactants and products
III.changing the reaction coefficients
A)I only
B)II only
C)III only
D)I and II only
E)I and III only

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
Addition of reactants to a chemical reaction in solution or gas phase at equilibrium results in ________
A)an increase in K and a shift in equilibrium to produce more products.
B)an increase in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.
A)an increase in K and a shift in equilibrium to produce more products.
B)an increase in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
Identify whether or not perturbations A-D will affect the equilibrium concentrations of reactants and products in a gas-phase endothermic chemical reaction that involves one or more gases. If all have an effect on the equilibrium concentrations, select response E. Otherwise, identify the one that does not affect the equilibrium concentrations.
A)adding reactants to a gas or solution reaction
B)removing products from a gas or solution reaction
C)decreasing the temperature
D)increasing pressure by adding an inert gas to a reaction in the gas phase
E)All of the above are perturbations to chemical equilibrium.
A)adding reactants to a gas or solution reaction
B)removing products from a gas or solution reaction
C)decreasing the temperature
D)increasing pressure by adding an inert gas to a reaction in the gas phase
E)All of the above are perturbations to chemical equilibrium.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Increasing the temperature of an exothermic reaction results in ________
A)more products and fewer reactants.
B)more reactants and fewer products.
C)more reactants and products.
D)fewer reactants and products.
E)no change in the quantities of reactants and products.
A)more products and fewer reactants.
B)more reactants and fewer products.
C)more reactants and products.
D)fewer reactants and products.
E)no change in the quantities of reactants and products.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following occurs when reactants are added to a chemical reaction in solution or the gas phase at equilibrium?
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
Addition of products to a chemical reaction in solution or gas phase at equilibrium results in ________
A)an increase in K and a shift in equilibrium to produce more reactants.
B)no change in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.
A)an increase in K and a shift in equilibrium to produce more reactants.
B)no change in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
How would an increase in temperature affect the following equilibrium? N2(g) 3H2(g) 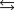 2NH3(g)
2NH3(g)  H° 92.0 kJ/mol
H° 92.0 kJ/mol
A)More product would form and K would increase.
B)More product would form but K would remain constant.
C)K would be unaffected by a temperature change.
D)More reactants would form but K would remain constant.
E)More reactants would form and K would decrease.
 2NH3(g)
2NH3(g)  H° 92.0 kJ/mol
H° 92.0 kJ/molA)More product would form and K would increase.
B)More product would form but K would remain constant.
C)K would be unaffected by a temperature change.
D)More reactants would form but K would remain constant.
E)More reactants would form and K would decrease.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
How would an increase in temperature affect the following equilibrium? H2O(l )  H2(g)
H2(g) 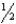 O2(g)
O2(g)  H 482.0 kJ/mol
H 482.0 kJ/mol
A)More product would form and K would increase.
B)More product would form but K would remain constant.
C)K would be unaffected by a temperature change.
D)More reactants would form but K would remain constant.
E)More reactants would form and K would decrease.
 H2(g)
H2(g) 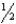 O2(g)
O2(g)  H 482.0 kJ/mol
H 482.0 kJ/molA)More product would form and K would increase.
B)More product would form but K would remain constant.
C)K would be unaffected by a temperature change.
D)More reactants would form but K would remain constant.
E)More reactants would form and K would decrease.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


