Deck 15: Acidbase Equilibria: Proton Transfer in Biological Systems
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/122
Play
Full screen (f)
Deck 15: Acidbase Equilibria: Proton Transfer in Biological Systems
1
The base ionization constant Kb describes which of the following reactions for a weak base, B, in aqueous solution?
A)B H
 BH
BH
B)B H3O BH H2O
BH H2O
C)B H2O BH OH
BH OH
D)B OH BH O2
BH O2
E)BH OH B H2O
B H2O
A)B H
 BH
BHB)B H3O
 BH H2O
BH H2OC)B H2O
 BH OH
BH OHD)B OH
 BH O2
BH O2E)BH OH
 B H2O
B H2OB H2O  BH OH
BH OH
 BH OH
BH OH 2
In the following reaction in aqueous solution, the base reactant is ________ and its conjugate acid product is ________. HCOOH C5H5N  HCOO C5H5NH
HCOO C5H5NH
A)HCOOH; HCOO
B)C5H5N; HCOO
C)HCOOH; C5H5NH
D)C5H5N; C5H5NH
E)C5H5N; OH
 HCOO C5H5NH
HCOO C5H5NHA)HCOOH; HCOO
B)C5H5N; HCOO
C)HCOOH; C5H5NH
D)C5H5N; C5H5NH
E)C5H5N; OH
C5H5N; C5H5NH
3
Solutions of each of the hypothetical bases in the following table are prepared with an initial concentration of 0.100 M. Which of the four solutions will have the lowest pH and be least basic?

A)A
B)B
C)C
D)D
E)All will have the same pH because the concentrations are the same.

A)A
B)B
C)C
D)D
E)All will have the same pH because the concentrations are the same.
A
4
Which sketch best represents the qualitative molecular view of an aqueous solution of sodium hydroxide? (Water molecules are not shown explicitly.) 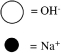
A)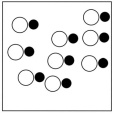
B)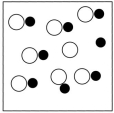
C)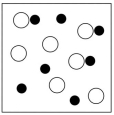
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
5
In the following reaction in aqueous solution, the acid reactant is ________ and its conjugate base product is ________. CH3NH2 HSO4  CH3NH3 SO42
CH3NH3 SO42
A)CH3NH2; CH3NH3
B)CH3NH2; SO42
C)HSO4; CH3NH3
D)HSO4; SO42
E)HSO4; H3O
 CH3NH3 SO42
CH3NH3 SO42A)CH3NH2; CH3NH3
B)CH3NH2; SO42
C)HSO4; CH3NH3
D)HSO4; SO42
E)HSO4; H3O

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a strong acid?
A)HNO3
B)H2S
C)HNO2
D)HCO3
E)HOCl
A)HNO3
B)H2S
C)HNO2
D)HCO3
E)HOCl

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the conjugate acid of the hydrogen phosphate ion, HPO42?
A)H3PO4
B)H2PO4
C)HPO42
D)PO43
E)H3O
A)H3PO4
B)H2PO4
C)HPO42
D)PO43
E)H3O

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
8
Ammonia (NH3) acts as a weak base in aqueous solution. What is the acid that reacts with this base when ammonia is dissolved in water?
A)None, there are no acids in pure water.
B)H2O
C)NH4
D)trick question, because no acids are present, ammonia cannot act as a base
E)oxygen that always is dissolved in water
A)None, there are no acids in pure water.
B)H2O
C)NH4
D)trick question, because no acids are present, ammonia cannot act as a base
E)oxygen that always is dissolved in water

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
9
The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid) ________
A)HA OH H2O A.
H2O A.
B)HA H2O H3O A.
H3O A.
C)HA H3O H2A H2O.
H2A H2O.
D)HA H2A H2A HA.
H2A HA.
E)H3O A HA H2O.
HA H2O.
A)HA OH
 H2O A.
H2O A.B)HA H2O
 H3O A.
H3O A.C)HA H3O
 H2A H2O.
H2A H2O.D)HA H2A
 H2A HA.
H2A HA.E)H3O A
 HA H2O.
HA H2O.
Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following is a conjugate acid-base pair?
A)NH3 and NH4
B)H3O and OH
C)NH2 and NH4
D)H2O and O2
E)NaF and F
A)NH3 and NH4
B)H3O and OH
C)NH2 and NH4
D)H2O and O2
E)NaF and F

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
11
Which sketch best represents the qualitative molecular view of an aqueous solution of nitrous acid? (Water molecules are not shown explicitly.) 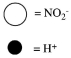
A)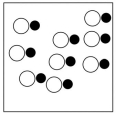
B)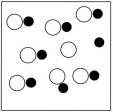
C)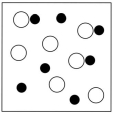
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
12
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples). The pKa values are LA 3.88, OA 1.23, and MA 3.40. Which list has these acids in order of decreasing acid strength?
A)LA OA MA
B)LA MA OA
C)OA MA LA
D)OA LA MA
E)MA LA OA
A)LA OA MA
B)LA MA OA
C)OA MA LA
D)OA LA MA
E)MA LA OA

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the conjugate base of the hydrogen carbonate ion, HCO3?
A)H2CO3
B)HCO3
C)CO32
D)OH
E)H3CO3
A)H2CO3
B)HCO3
C)CO32
D)OH
E)H3CO3

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following is not a strong base?
A)lithium hydroxide, LiOH
B)sodium hydroxide, NaOH
C)potassium hydroxide, KOH
D)calcium hydroxide, Ca(OH)2
E)ammonium hydroxide, NH4OH
A)lithium hydroxide, LiOH
B)sodium hydroxide, NaOH
C)potassium hydroxide, KOH
D)calcium hydroxide, Ca(OH)2
E)ammonium hydroxide, NH4OH

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following is not a strong acid?
A)nitric acid, HNO3
B)sulfuric acid, H2SO4
C)carbonic acid, H2CO3
D)hydrochloric acid, HCl
E)perchloric acid, HClO4
A)nitric acid, HNO3
B)sulfuric acid, H2SO4
C)carbonic acid, H2CO3
D)hydrochloric acid, HCl
E)perchloric acid, HClO4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following is not a conjugate acid-base pair?
A)NH3 and NH2
B)HNO3 and HNO2
C)HI and I
D)H2PO4 and HPO42
E)H2O and OH
A)NH3 and NH2
B)HNO3 and HNO2
C)HI and I
D)H2PO4 and HPO42
E)H2O and OH

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following is a strong acid?
A)nitrous acid, HNO2
B)sulfurous acid, H2SO3
C)carbonic acid, H2CO3
D)hydrofluoric acid, HF
E)perchloric acid, HClO4
A)nitrous acid, HNO2
B)sulfurous acid, H2SO3
C)carbonic acid, H2CO3
D)hydrofluoric acid, HF
E)perchloric acid, HClO4

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following is a conjugate acid-base pair?
A)NaF and F
B)HNO3 and HNO2
C)HI and I
D)NH4 and NH2
E)H2O and H2O2
A)NaF and F
B)HNO3 and HNO2
C)HI and I
D)NH4 and NH2
E)H2O and H2O2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
19
In the following reaction in aqueous solution, the acid reactant is ________ and its conjugate base product is ________. CH3COOH NH3  CH3COO NH4
CH3COO NH4
A)CH3COOH; CH3COO
B)CH3COOH; NH4
C)NH3; CH3COO
D)NH3; NH4
E)CH3COOH; H3O
 CH3COO NH4
CH3COO NH4A)CH3COOH; CH3COO
B)CH3COOH; NH4
C)NH3; CH3COO
D)NH3; NH4
E)CH3COOH; H3O

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is not a conjugate acid-base pair?
A)NH3 and NH4
B)H3O and OH
C)H2PO4 and HPO42
D)HS and H2S
E)NH3 and NH2
A)NH3 and NH4
B)H3O and OH
C)H2PO4 and HPO42
D)HS and H2S
E)NH3 and NH2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
21
A substance that can act as both an acid and base is ________
A)amphibious.
B)amphiprotic.
C)bacidic.
D)androgynous.
E)acibasic.
A)amphibious.
B)amphiprotic.
C)bacidic.
D)androgynous.
E)acibasic.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
22
When [H] 1.0 107 M in water at 25C, then ________
A)pH 1.
B)pH 107.
C)[OH] 1.0 107 M.
D)[OH] 1.0 107 M.
E)[OH] 0 M.
A)pH 1.
B)pH 107.
C)[OH] 1.0 107 M.
D)[OH] 1.0 107 M.
E)[OH] 0 M.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
23
Which one A-D is not related to the water autoionization constant, Kw?
A)[H3O] [OH]
B)1.0 1014 at 25C
C)2 H2O![<strong>Which one A-D is not related to the water autoionization constant, K<sub>w</sub>?</strong> A)[H<sub>3</sub>O<font face=symbol><sup></sup></font>] [OH<font face=symbol><sup></sup></font>] B)1.0 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>14</sup> at 25<font face=symbol></font>C C)2<sup> </sup>H<sub>2</sub>O H<sub>3</sub>O<font face=symbol><sup></sup></font> <font face=symbol></font> OH<font face=symbol><sup></sup></font> D)pH <font face=symbol></font> 7 at 25<font face=symbol></font>C E)A-D are all related to K<sub>w</sub>.](https://storage.examlex.com/TB3835/11eaa952_9b45_16a8_ae90_65a83aeac875_TB3835_11.jpg) H3O OH
H3O OH
D)pH 7 at 25C
E)A-D are all related to Kw.
A)[H3O] [OH]
B)1.0 1014 at 25C
C)2 H2O
![<strong>Which one A-D is not related to the water autoionization constant, K<sub>w</sub>?</strong> A)[H<sub>3</sub>O<font face=symbol><sup></sup></font>] [OH<font face=symbol><sup></sup></font>] B)1.0 <font face=symbol></font> 10<font face=symbol><sup></sup></font><sup>14</sup> at 25<font face=symbol></font>C C)2<sup> </sup>H<sub>2</sub>O H<sub>3</sub>O<font face=symbol><sup></sup></font> <font face=symbol></font> OH<font face=symbol><sup></sup></font> D)pH <font face=symbol></font> 7 at 25<font face=symbol></font>C E)A-D are all related to K<sub>w</sub>.](https://storage.examlex.com/TB3835/11eaa952_9b45_16a8_ae90_65a83aeac875_TB3835_11.jpg) H3O OH
H3O OHD)pH 7 at 25C
E)A-D are all related to Kw.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
24
Sometimes liquid ammonia, NH3, is used as a solvent rather than water. Which expression defines the ammonia autoionization counterpart of Kw?
A)[H3O][OH]
B)[NH3][NH4]
C)[NH2][NH4]
D)[H3O][NH2]
E)[NH4][OH]
A)[H3O][OH]
B)[NH3][NH4]
C)[NH2][NH4]
D)[H3O][NH2]
E)[NH4][OH]

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
25
What is the actual concentration of the molecular form of HF in a 1.0 M HF solution given that Ka of HF is 6.8 104?
A)2.6 102 M
B)0.97 M
C)1.59 M
D)6.8 104 M
E)1.0 M
A)2.6 102 M
B)0.97 M
C)1.59 M
D)6.8 104 M
E)1.0 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
26
Use the following acid ionization constants to identify the correct decreasing order of base strengths for the conjugate bases. HF
Ka 7.2 104
HNO2
Ka 4.5 104
HCN
Ka 6.2 1010
A)CN NO2 F
B)NO2 F CN
C)F CN NO2
D)F NO2 CN
E)NO2 CN F
Ka 7.2 104
HNO2
Ka 4.5 104
HCN
Ka 6.2 1010
A)CN NO2 F
B)NO2 F CN
C)F CN NO2
D)F NO2 CN
E)NO2 CN F

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
27
Three common weak bases are phosphate (P; PO43, pKb 1.3), carbonate (C; CO32, pKb 3.7), and acetate (A; CH3COO, pKb 9.3). Which response has these bases listed in order of increasing strength?
A)P C A
B)C P A
C)A C P
D)C A P
E)P A C
A)P C A
B)C P A
C)A C P
D)C A P
E)P A C

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
28
A cup of coffee has a hydroxide ion concentration of 1.0 1010 M. What is the pH of this coffee?
A)1.0 104
B)4
C)10
D)7
E)(10)
A)1.0 104
B)4
C)10
D)7
E)(10)

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
29
Which statement A-D is not correct? Pure water at 25C has ________
A)Kw 1.0 1014.
B)pOH 7.
C)[H3O] [OH].
D)pH 7.
E)A-D are all correct.
A)Kw 1.0 1014.
B)pOH 7.
C)[H3O] [OH].
D)pH 7.
E)A-D are all correct.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
30
When [H] 4.0 109 M in water at 25C, then ________
A)pH 9.40.
B)pH 7.00.
C)pH 8.40.
D)pH 8.40.
E)pH 9.40.
A)pH 9.40.
B)pH 7.00.
C)pH 8.40.
D)pH 8.40.
E)pH 9.40.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
31
Pure water at any temperature has ________
A)a pH less than 7.
B)a pOH more than 7.
C)[H3O] [OH].
D)pH 7.
E)no hydronium ions in it.
A)a pH less than 7.
B)a pOH more than 7.
C)[H3O] [OH].
D)pH 7.
E)no hydronium ions in it.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
32
A solution with a pOH of 4.3 has a [H] of ________
A)6.8 109 M.
B)3.2 104 M.
C)4.8 105 M.
D)2.0 1010 M.
E)4.3 M.
A)6.8 109 M.
B)3.2 104 M.
C)4.8 105 M.
D)2.0 1010 M.
E)4.3 M.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
33
The pH of a popular soft drink is 3.4; what is its hydronium ion concentration?
A)5.0 104 M
B)4.0 104 M
C)2.5 103 M
D)1.0 107 M
E)5.0 105 M
A)5.0 104 M
B)4.0 104 M
C)2.5 103 M
D)1.0 107 M
E)5.0 105 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
34
Which expression defines the autoionization constant for water, Kw?
A)[H3O][OH]
B)[H2O][H3O]
C)[OH][H2O]
D)[H4O2][O2]
E)[H2O][H2O]
A)[H3O][OH]
B)[H2O][H3O]
C)[OH][H2O]
D)[H4O2][O2]
E)[H2O][H2O]

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
35
A solution with a pH of 9.50 has a pOH of ________
A)9.50.
B)0.50.
C)4.50.
D)23.5.
E)19.0.
A)9.50.
B)0.50.
C)4.50.
D)23.5.
E)19.0.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
36
When pure water autoionizes, which ions are produced?
A)O2, OH, H3O, and H2O
B)OH and H3O
C)O2 and H4O2
D)H and OH
E)2H and O2
A)O2, OH, H3O, and H2O
B)OH and H3O
C)O2 and H4O2
D)H and OH
E)2H and O2

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
37
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples). The pKa values are LA 3.88, OA 1.23, and MA 3.40. Which list has the conjugate bases of these acids in order of decreasing strength?
A)lactate oxalate malate
B)oxalate malate lactate
C)lactate malate oxalate
D)oxalate lactate malate
E)malate lactate oxalate
A)lactate oxalate malate
B)oxalate malate lactate
C)lactate malate oxalate
D)oxalate lactate malate
E)malate lactate oxalate

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
38
A solution with a pOH of 6.92 has an [OH] concentration of ________
A)1.20 107 M.
B)9.2 106 M.
C)6.8 106 M.
D)7.08 M.
E)6.92 M.
A)1.20 107 M.
B)9.2 106 M.
C)6.8 106 M.
D)7.08 M.
E)6.92 M.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
39
A solution with a pH of 2.50 has an [OH] of ________
A)3.16 102.
B)3.16 1012.
C)3.16 103.
D)3.16 1016.
E)3.16 1011.
A)3.16 102.
B)3.16 1012.
C)3.16 103.
D)3.16 1016.
E)3.16 1011.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
40
The hydronium ion concentration of a dilute solution of vinegar is 1.45 105. What is the pH of this solution?
A)5.7
B)(4.8)
C)4.8
D)(5.7)
E)7.0
A)5.7
B)(4.8)
C)4.8
D)(5.7)
E)7.0

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
41
The concentration of acetic acid (pKa 4.75) in vinegar is about 1.0 M. With this information, what do you predict the pH of vinegar to be?
A)4.75
B)2.4
C)4.0 103
D)7.0
E)5.35
A)4.75
B)2.4
C)4.0 103
D)7.0
E)5.35

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
42
What is the pOH of a 0.125 M solution of hydroxylamine? The Kb value for hydroxylamine is 1.1 108.
A)4.43
B)3.47
C)5.57
D)10.53
E)9.57
A)4.43
B)3.47
C)5.57
D)10.53
E)9.57

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
43
In evaluating the pH of an aqueous weak acid solution, ________ usually can be ignored.
A)the concentration of the weak acid
B)the concentration of hydronium ion produced by the autoionization of water
C)the reaction of the weak acid with water
D)the concentration of the ionized hydronium ion
E)the concentration of the conjugate base
A)the concentration of the weak acid
B)the concentration of hydronium ion produced by the autoionization of water
C)the reaction of the weak acid with water
D)the concentration of the ionized hydronium ion
E)the concentration of the conjugate base

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
44
The acidic ingredient in vinegar is acetic acid. The pH of vinegar is around 2.4, and the molar concentration of acetic acid in vinegar is around 0.85 M. Based on this information, determine the value of the acid ionization constant, Ka, for acetic acid.
A)2.5 105
B)5.0 105
C)4.7 103
D)1.9 105
E)7.4 103
A)2.5 105
B)5.0 105
C)4.7 103
D)1.9 105
E)7.4 103

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
45
What is the actual concentration of molecular NH3 in a 0.200 M solution of ammonia? The Kb value for ammonia is 1.80 105.
A)0.200 M
B)0.198 M
C)1.80 105 M
D)1.90 103 M
E)3.6 106 M
A)0.200 M
B)0.198 M
C)1.80 105 M
D)1.90 103 M
E)3.6 106 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
46
Diethylamine ((CH3CH2) NH2) is a weakly basic compound. Calculate the Kb for diethylamine if a 0.127 M solution is 9.61% ionized.
A)1.8 105
B)4.4 104
C)5.6 104
D)1.3 103
E)3.8 1010
A)1.8 105
B)4.4 104
C)5.6 104
D)1.3 103
E)3.8 1010

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
47
What is the concentration of the acetate ion in a 1.00 M CH3COOH solution given that the Ka of CH3COOH is 1.8 105?
A)4.20 103 M
B)0.996 M
C)1.00 M
D)2.84 104 M
E)5.62 105 M
A)4.20 103 M
B)0.996 M
C)1.00 M
D)2.84 104 M
E)5.62 105 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
48
Methylamine (CH3NH2) is a weakly basic compound. Calculate the Kb for methylamine if a 0.253 M solution is 4.07% ionized.
A)2.29 103
B)4.37 104
C)5.72
D)4.24 102
E)4.19 104
A)2.29 103
B)4.37 104
C)5.72
D)4.24 102
E)4.19 104

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
49
What is the concentration of ammonium ion in a 0.150 M solution of ammonia? The Kb value for ammonia is 1.80 105.
A)0.146 M
B)0.148 M
C)2.34 105 M
D)1.63 103 M
E)4.20 103 M
A)0.146 M
B)0.148 M
C)2.34 105 M
D)1.63 103 M
E)4.20 103 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
50
When values of Ka are small (e.g., 1 105) and concentrations of weak acids [HA] are relatively large (e.g., 0.10 M), the hydronium ion concentration of the solution can be calculated using which expression?
A)[H] Ka
B)[H] Ka[HA]
C)[H] (Ka[HA])1/2
D)[H] KaKb[HA]
E)[H] Ka[HA]/[A]
A)[H] Ka
B)[H] Ka[HA]
C)[H] (Ka[HA])1/2
D)[H] KaKb[HA]
E)[H] Ka[HA]/[A]

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
51
What is the concentration of [OH] in a 0.20 M solution of ammonia? The Kb value for ammonia is 1.8 105.
A)3.6 106 M
B)1.8 105 M
C)0.20 M
D)1.9 103 M
E)4.2 104 M
A)3.6 106 M
B)1.8 105 M
C)0.20 M
D)1.9 103 M
E)4.2 104 M

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pH of a 0.500 M solution of trimethylamine (pKb 4.13)?
A)2.22
B)11.8
C)0.00609
D)4.42
E)5.91
A)2.22
B)11.8
C)0.00609
D)4.42
E)5.91

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
53
What is the pH of a 0.030 M solution of oxalic acid? Ka for oxalic acid is 5.9 102.
A)1.66
B)1.37
C)2.78
D)12.67
E)12.34
A)1.66
B)1.37
C)2.78
D)12.67
E)12.34

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
54
Lactic acid is a major component of Ringer's solution, which is used as an intravenous fluid to maintain fluid balance in trauma patients. The pH of a 0.100 M lactic acid solution is around 2.44. Based on this information, determine the value of the acid ionization constant, Ka, for lactic acid.
A)1.2 102
B)1.4 104
C)4.7 103
D)1.8 104
E)7.4 103
A)1.2 102
B)1.4 104
C)4.7 103
D)1.8 104
E)7.4 103

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
55
What is the hydronium ion concentration of a 0.010 M solution of acetic acid? Ka for acetic acid is 1.8 105.
A)1.8 103
B)1.8 105
C)1.0 102
D)1.8 107
E)4.2 104
A)1.8 103
B)1.8 105
C)1.0 102
D)1.8 107
E)4.2 104

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
56
Formic acid is a weak acid naturally occurring in certain species of ants. Calculate the acid ionization constant for formic acid if a 0.213 M solution is 2.86% ionized. The abbreviated structural formula for formic acid is HCOOH.
A)7.2 104
B)6.4 104
C)1.8 104
D)1.9 105
E)3.5 108
A)7.2 104
B)6.4 104
C)1.8 104
D)1.9 105
E)3.5 108

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
57
A 0.100 M solution of a monoprotic weak acid has a pH of 3.00. What is the pKa of this acid?
A)5.00
B)0.999
C)3.00
D)9.99
E)6.00
A)5.00
B)0.999
C)3.00
D)9.99
E)6.00

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
58
Boric acid frequently is used as an eyewash to treat eye infections. The pH of a 0.050 M solution of boric acid is 5.28. What is the value of the boric acid ionization constant, Ka?
A)5.25 106
B)5.51 1010
C)5.43 108
D)5.79 104
E)5.33 1012
A)5.25 106
B)5.51 1010
C)5.43 108
D)5.79 104
E)5.33 1012

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
59
Butanoic acid contributes to the rancid odor of spoiled butter. Calculate the acid ionization constant for butanoic acid if a 0.155 M solution is 1.15% ionized. The abbreviated structural formula for butanoic acid is CH3CH2CH2COOH.
A)5.1 103
B)1.8 103
C)1.2 102
D)2.1 105
E)1.5 105
A)5.1 103
B)1.8 103
C)1.2 102
D)2.1 105
E)1.5 105

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
60
The first disinfectant used by Joseph Lister was called carbolic acid. This substance now is known as phenol, C6H5OH (pKa 10.0). What is the pH of a 0.10 M solution of phenol?
A)3.5
B)10.0
C)6.5
D)5.5
E)4.5
A)3.5
B)10.0
C)6.5
D)5.5
E)4.5

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
61
Sodium hypochlorite is a common ingredient in household bleach. What is the pH of this bleach if it contains 5% NaOCl by mass? (pKa of HOCl 7.46)
A)9
B)11
C)4
D)8
E)7
A)9
B)11
C)4
D)8
E)7

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
62
The degree of ionization of a strong acid is ________
A)dependent on the concentration of the acid.
B)between 1 and 10%.
C)between 10 and 100%.
D)100%.
E)dependent on which strong acid it is.
A)dependent on the concentration of the acid.
B)between 1 and 10%.
C)between 10 and 100%.
D)100%.
E)dependent on which strong acid it is.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. HCl, H2S, PH3
A)HCl H2S
B)HCl PH3
C)H2S HCl
D)H2S PH3
E)PH3 HCl
A)HCl H2S
B)HCl PH3
C)H2S HCl
D)H2S PH3
E)PH3 HCl

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
64
In each of the following, the stronger acid is identified and an explanation is given. All except one are correct statements. Which one is not correct?
A)HCl HF because the HF bond energy is larger than the HCl bond energy.
B)HCO3 H2CO3 because the negative charge stabilizes the loss of a proton.
C)CCl3COOH CH3COOH because electronegative substituents stabilize the conjugate base.
D)HBrO3 HBrO2 because of the additional electronegative oxygen atom.
E)ClOH BrOH because Cl is more electronegative than Br.
A)HCl HF because the HF bond energy is larger than the HCl bond energy.
B)HCO3 H2CO3 because the negative charge stabilizes the loss of a proton.
C)CCl3COOH CH3COOH because electronegative substituents stabilize the conjugate base.
D)HBrO3 HBrO2 because of the additional electronegative oxygen atom.
E)ClOH BrOH because Cl is more electronegative than Br.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
65
Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. H2O, H2S, H2Se
A)H2Se H2S
B)H2Se H2O
C)H2S H2Se
D)H2O H2Se
E)H2O H2S
A)H2Se H2S
B)H2Se H2O
C)H2S H2Se
D)H2O H2Se
E)H2O H2S

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
66
What is the hydronium ion concentration of a 0.200 M solution of methylamine? The Kb value for methylamine is 4.38 104.
A)9.14 103
B)1.09 1012
C)5.24 1014
D)1.00 107
E)1.91 101
A)9.14 103
B)1.09 1012
C)5.24 1014
D)1.00 107
E)1.91 101

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
67
Which one of the following statements is not correct?
A)HIO is a stronger acid than HClO.
B)HClO is a stronger acid than HBrO.
C)HClO3 is a stronger acid than HClO2.
D)HPO42 is a weaker acid than H2PO4.
E)H2SO4 is a stronger acid than H2SO3.
A)HIO is a stronger acid than HClO.
B)HClO is a stronger acid than HBrO.
C)HClO3 is a stronger acid than HClO2.
D)HPO42 is a weaker acid than H2PO4.
E)H2SO4 is a stronger acid than H2SO3.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pH of a 0.200 M solution of methylamine? The Kb value for methylamine is 4.38 104.
A)2.04
B)(1.33)
C)10.53
D)15.33
E)11.96
A)2.04
B)(1.33)
C)10.53
D)15.33
E)11.96

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
69
The analysis label on a 500 mL bottle of Fiji natural artesian water reports pH 7.5 and 140 mg of bicarbonates, HCO3. Calculate the pH expected as a result of the bicarbonates to see if these two numbers are consistent with each other. (Kb 2.4 108 for HCO3)
A)7.5
B)5.0
C)9.6
D)4.4
E)9.0
A)7.5
B)5.0
C)9.6
D)4.4
E)9.0

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
70
The degree of ionization of a weak acid ________
I. varies with the concentration of the acid.
II. depends on which weak acid it is.
III. is 100%.
IV. is greater than 50% but less than 100%.
A)I only
B)II only
C)III only
D)both I and II
E)IV only
I. varies with the concentration of the acid.
II. depends on which weak acid it is.
III. is 100%.
IV. is greater than 50% but less than 100%.
A)I only
B)II only
C)III only
D)both I and II
E)IV only

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
71
The pH of an aniline solution is 8.91, and the solution has a concentration of 0.17 M. The pKb of aniline is 9.42. What is the percent ionization of aniline?
A)1.2%
B)0.50%
C)2.1 103%
D)4.7 103%
E)6.3 105%
A)1.2%
B)0.50%
C)2.1 103%
D)4.7 103%
E)6.3 105%

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
72
A solution of the weak acid HF and a solution of the strong acid HCl have the same pH. Which solution will require the most sodium hydroxide, NaOH, to neutralize?
A)HCl, because it is a strong acid and dissociates completely.
B)HF, because its concentration is larger.
C)Both will require the same amount because the concentrations are equal.
D)Both will require the same amount because the H3O concentrations are the same.
E)HCl, because the stronger acid has the higher concentration.
A)HCl, because it is a strong acid and dissociates completely.
B)HF, because its concentration is larger.
C)Both will require the same amount because the concentrations are equal.
D)Both will require the same amount because the H3O concentrations are the same.
E)HCl, because the stronger acid has the higher concentration.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
73
Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. H2Te, H2S, H2Se
A)H2Se H2S
B)H2S H2Te
C)H2S H2Se
D)H2Te H2Se
E)H2Te H2S
A)H2Se H2S
B)H2S H2Te
C)H2S H2Se
D)H2Te H2Se
E)H2Te H2S

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
74
Which ending to the statement is not correct? The weak acid HY will be stronger than the weak acid HZ if ________
A)more Lewis resonance structures can be written for the Y group than for the Z group.
B)Y is more electronegative than Z.
C)the Y group has more oxygen atoms than the Z group.
D)the HY bond is weaker than the HZ bond and other things are about the same.
E)the Y group contains Br rather than Cl, which is in the Z group.
A)more Lewis resonance structures can be written for the Y group than for the Z group.
B)Y is more electronegative than Z.
C)the Y group has more oxygen atoms than the Z group.
D)the HY bond is weaker than the HZ bond and other things are about the same.
E)the Y group contains Br rather than Cl, which is in the Z group.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
75
The pH of vinegar is 2.4, and the acetic acid in vinegar has a concentration of about 0.85 M. The pKa of acetic acid is 4.75. What is the percent ionization of acetic acid in vinegar?
A)2.8%
B)4.7%
C)0.47%
D)0.21%
E)0.021%
A)2.8%
B)4.7%
C)0.47%
D)0.21%
E)0.021%

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
76
The pH of an ammonia solution is 11.6, and the solution has a concentration of about 0.20 M. The pKb of ammonia is 4.75. What is the percent ionization of ammonia?
A)2.0%
B)2.3%
C)1.2%
D)0.95%
E)5.4%
A)2.0%
B)2.3%
C)1.2%
D)0.95%
E)5.4%

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
77
Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. HOCl, HOBr, HOI
A)HOBr HOCl
B)HOBr HOI
C)HOCl HOBr
D)HOCl HOI
E)HOI HOCl
A)HOBr HOCl
B)HOBr HOI
C)HOCl HOBr
D)HOCl HOI
E)HOI HOCl

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
78
Vitamin C, which is ascorbic acid, is a diprotic acid with 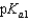 5.00 and
5.00 and 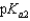 11.3. What is the pH of a 0.125 M solution of ascorbic acid?
11.3. What is the pH of a 0.125 M solution of ascorbic acid?
A)2.95
B)3.05
C)5.00
D)6.10
E)3.54
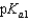 5.00 and
5.00 and 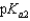 11.3. What is the pH of a 0.125 M solution of ascorbic acid?
11.3. What is the pH of a 0.125 M solution of ascorbic acid?A)2.95
B)3.05
C)5.00
D)6.10
E)3.54

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
79
The degree of ionization ________
A)increases with increasing concentration of a weak acid.
B)decreases with increasing concentration of a weak acid.
C)does not change with changing concentration of a weak acid.
D)is not related to the concentration of a weak acid.
E)is independent of the composition of the weak acid.
A)increases with increasing concentration of a weak acid.
B)decreases with increasing concentration of a weak acid.
C)does not change with changing concentration of a weak acid.
D)is not related to the concentration of a weak acid.
E)is independent of the composition of the weak acid.

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck
80
Identify the weakest and strongest acids in the group below. The weakest acid is listed first in the responses. HCl, HBr, HI
A)HCl HI
B)HCl HBr
C)HBr HCl
D)HBr HI
E)HI HCl
A)HCl HI
B)HCl HBr
C)HBr HCl
D)HBr HI
E)HI HCl

Unlock Deck
Unlock for access to all 122 flashcards in this deck.
Unlock Deck
k this deck


