Deck 12: Solids: Crystals, Alloys, and Polymers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 12: Solids: Crystals, Alloys, and Polymers
1
Which of the following is true regarding the attractive force that holds sodium chloride in the solid state?
I.It is electrostatic.
II.It is termed ionic bonding.
III.It depends on the distance between the sodium and chloride.
IV.It only operates between adjacent sodium and chloride ions.
A)I and II only
B)II and III only
C)I, II, and III only
D)II and IV only
E)I-IV are all true statements.
I.It is electrostatic.
II.It is termed ionic bonding.
III.It depends on the distance between the sodium and chloride.
IV.It only operates between adjacent sodium and chloride ions.
A)I and II only
B)II and III only
C)I, II, and III only
D)II and IV only
E)I-IV are all true statements.
I, II, and III only
2
Quartz, a form of SiO2, which contains an ordered array of neutral, covalently bonded nonmetal units, is an example of ________
A)a molecular solid.
B)a metallic solid.
C)an alloy.
D)an ionic solid.
E)an amorphous solid.
A)a molecular solid.
B)a metallic solid.
C)an alloy.
D)an ionic solid.
E)an amorphous solid.
a molecular solid.
3
A cubic closest-packed structure has hexagonally arranged layers of atoms stacking in the series ________
A)ababab
B)abcabcabc.
C)abcbabcbabcba.
D)abacabacaba.
E)aaaaaa.
A)ababab
B)abcabcabc.
C)abcbabcbabcba.
D)abacabacaba.
E)aaaaaa.
abcabcabc.
4
At a historic Civil War battleground, a stack of cannonballs looked like the picture below on the far left. Removing the top cannonball resulted in the middle view, and removing the next layer resulted in the view on the right. What sort of packing was used in stacking the cannonballs? 
A)cannonball closest-packed
B)hexagonal closest-packed
C)cubic closest-packed
D)random-packed
E)body-centered closest-packed

A)cannonball closest-packed
B)hexagonal closest-packed
C)cubic closest-packed
D)random-packed
E)body-centered closest-packed

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following contribute to the arrangement of ions in the unit cells of an ionic solid?
I.the empirical formula
II.the relative radii of the ions
III.the shape of polyatomic ions
A)I and II only
B)II and III only
C)I and III only
D)I only
E)I, II, and III
I.the empirical formula
II.the relative radii of the ions
III.the shape of polyatomic ions
A)I and II only
B)II and III only
C)I and III only
D)I only
E)I, II, and III

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
What type of crystal structure produces an aaaaaa layering pattern?
A)hexagonal
B)face-centered cubic
C)simple cubic
D)body-centered cubic
E)hexagonal or body-centered cubic
A)hexagonal
B)face-centered cubic
C)simple cubic
D)body-centered cubic
E)hexagonal or body-centered cubic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
What type of crystal structure can produce an ababab layering pattern?
A)hexagonal
B)face-centered cubic
C)simple cubic
D)body-centered cubic
E)hexagonal or body-centered cubic
A)hexagonal
B)face-centered cubic
C)simple cubic
D)body-centered cubic
E)hexagonal or body-centered cubic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement A-D does not describe a simple cubic unit cell?
A)It results from square packing of atoms in layers.
B)The atoms lie at the corners of a cube.
C)Each atom has 6 nearest neighbors.
D)The stacking pattern can be represented by aaaa.
E)Statements A-D all correctly describe a simple cubic unit cell.
A)It results from square packing of atoms in layers.
B)The atoms lie at the corners of a cube.
C)Each atom has 6 nearest neighbors.
D)The stacking pattern can be represented by aaaa.
E)Statements A-D all correctly describe a simple cubic unit cell.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
In the sodium chloride unit cell, the chloride ions form a cube in which each side is arranged like the following figure. The circles represent the positions of the chloride ions on one square face of the cube. All the other faces are the same. What is the name of this unit cell? 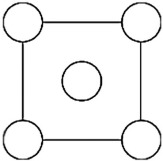
A)simple cubic
B)chloride-centered cubic
C)face-centered cubic
D)x-face cubic
E)body-centered cubic

A)simple cubic
B)chloride-centered cubic
C)face-centered cubic
D)x-face cubic
E)body-centered cubic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
Which is not true about a crystallographic unit cell?
A)It repeats throughout a crystalline structure in three dimensions.
B)It fills all the space in the crystalline lattice.
C)Its dimensions can be measured with X-rays.
D)It has corners with 90° angles.
E)It represents the smallest repeating unit in the crystal.
A)It repeats throughout a crystalline structure in three dimensions.
B)It fills all the space in the crystalline lattice.
C)Its dimensions can be measured with X-rays.
D)It has corners with 90° angles.
E)It represents the smallest repeating unit in the crystal.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
The cubic closest-packed crystal structure has a ________ layering pattern that produces a ________ unit cell.
A)ababab; hexagonal
B)ababab; face-centered cubic
C)abcabcabc; hexagonal
D)abcabcabc; face-centered cubic
E)abcabcabc; body-centered cubic
A)ababab; hexagonal
B)ababab; face-centered cubic
C)abcabcabc; hexagonal
D)abcabcabc; face-centered cubic
E)abcabcabc; body-centered cubic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
The closest-packing of spheres (such as oranges, cannonballs, or atoms) has the spheres ________
A)arranged in layers with each sphere surrounded by 4 other spheres in the layer.
B)arranged in layers with each sphere surrounded by 3 other spheres in the layer.
C)arranged in a square pattern with a sphere at each corner and one in the center of the square.
D)arranged in a square pattern with a sphere at each corner of the square.
E)arranged in layers with each sphere surrounded by 6 other spheres in the layer.
A)arranged in layers with each sphere surrounded by 4 other spheres in the layer.
B)arranged in layers with each sphere surrounded by 3 other spheres in the layer.
C)arranged in a square pattern with a sphere at each corner and one in the center of the square.
D)arranged in a square pattern with a sphere at each corner of the square.
E)arranged in layers with each sphere surrounded by 6 other spheres in the layer.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
What type of packing best describes the layering pattern shown below? 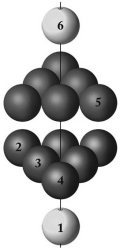
A)body-centered closest-packed
B)hexagonal closest-packed
C)random-packed
D)cubic closest-packed
E)cubic packing

A)body-centered closest-packed
B)hexagonal closest-packed
C)random-packed
D)cubic closest-packed
E)cubic packing

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
Ionic solids have ________ melting points, and are ________.
A)high; malleable
B)high; brittle
C)low; malleable
D)low; brittle
E)high; soft
A)high; malleable
B)high; brittle
C)low; malleable
D)low; brittle
E)high; soft

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
Identify the solid that has a high melting point, is hard and brittle, and conducts electricity when liquefied.
A)a network covalent solid
B)a metal
C)a covalent network
D)an ionic solid
E)an amorphous solid
A)a network covalent solid
B)a metal
C)a covalent network
D)an ionic solid
E)an amorphous solid

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
Which type of solid is held together by relatively weak dispersion forces?
A)ionic solid
B)metal
C)molecular solid
D)ceramic
E)semiconductor
A)ionic solid
B)metal
C)molecular solid
D)ceramic
E)semiconductor

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
The face-centered cubic structure is also known as ________
A)cubic closest-packed.
B)hexagonal closest-packed.
C)square closest-packed.
D)spherical closest-packed.
E)none of the above, as it is not a closest-packed pattern.
A)cubic closest-packed.
B)hexagonal closest-packed.
C)square closest-packed.
D)spherical closest-packed.
E)none of the above, as it is not a closest-packed pattern.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
A hexagonal closest-packed structure has hexagonally arranged layers of atoms stacking in the series ________
A)ababab
B)abcabcabc.
C)abcbabcbabcba.
D)abacabacaba.
E)aaaaaa.
A)ababab
B)abcabcabc.
C)abcbabcbabcba.
D)abacabacaba.
E)aaaaaa.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
The two types of closest-packed lattices are ________
A)cubic closest-packed and face-centered cubic.
B)cubic closest-packed and hexagonal closest-packed.
C)cubic closest-packed and random closest-packed.
D)cubic closest-packed and pyramidal closest-packed.
E)simple cubic and hexagonal closest-packed.
A)cubic closest-packed and face-centered cubic.
B)cubic closest-packed and hexagonal closest-packed.
C)cubic closest-packed and random closest-packed.
D)cubic closest-packed and pyramidal closest-packed.
E)simple cubic and hexagonal closest-packed.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
Pure solid metals ________
A)do not crystallize.
B)are amorphous.
C)often crystallize in closest-packed structures.
D)often crystallize in very complex unit cells.
E)are like liquids with the nuclei flowing through a sea of electrons.
A)do not crystallize.
B)are amorphous.
C)often crystallize in closest-packed structures.
D)often crystallize in very complex unit cells.
E)are like liquids with the nuclei flowing through a sea of electrons.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
Silver crystallizes with a face-centered cubic unit cell. The edge length is 407.7 pm. What is the volume of this unit cell?
A)7.68 107 pm3
B)6.78 107 pm3
C)8.67 107 pm3
D)9.87 107 pm3
E)3.88 107 pm3
A)7.68 107 pm3
B)6.78 107 pm3
C)8.67 107 pm3
D)9.87 107 pm3
E)3.88 107 pm3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following unit cells has the lowest packing efficiency?
A)simple cubic
B)face-centered cubic
C)body-centered cubic
D)both face-centered and body-centered cubic
E)Simple, face-centered, and body-centered cubic all have the same packing efficiency.
A)simple cubic
B)face-centered cubic
C)body-centered cubic
D)both face-centered and body-centered cubic
E)Simple, face-centered, and body-centered cubic all have the same packing efficiency.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
Copper crystallizes in a face-centered cubic pattern. How many copper atoms are in each unit cell?
A)2
B)4
C)8
D)12
E)14
A)2
B)4
C)8
D)12
E)14

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement does not describe a simple cubic unit cell?
A)It results from square packing of atoms in layers.
B)The atoms lie at the corners of a cube.
C)Each atom has 6 nearest neighbors.
D)The stacking pattern can be represented by aaaa.
E)Each unit cell contains 8 atoms.
A)It results from square packing of atoms in layers.
B)The atoms lie at the corners of a cube.
C)Each atom has 6 nearest neighbors.
D)The stacking pattern can be represented by aaaa.
E)Each unit cell contains 8 atoms.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
Iron crystallizes in a body-centered cubic pattern. How many iron atoms are in each unit cell?
A)1
B)2
C)4
D)8
E)9
A)1
B)2
C)4
D)8
E)9

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
Compare the packing efficiency of face-centered cubic copper and simple cubic polonium.
A)The efficiency of packing in the copper unit cell is higher.
B)The efficiency of packing in the polonium unit cell is higher.
C)The efficiencies of packing in the two lattices are the same.
D)Packing efficiencies cannot be defined for one or both of these.
E)There is no way to compare without further information.
A)The efficiency of packing in the copper unit cell is higher.
B)The efficiency of packing in the polonium unit cell is higher.
C)The efficiencies of packing in the two lattices are the same.
D)Packing efficiencies cannot be defined for one or both of these.
E)There is no way to compare without further information.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
An atom in a particular unit cell has 12 nearest neighbor atoms around it. Which unit cell(s) are possible?
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following unit cells has the highest packing efficiency?
A)simple cubic
B)face-centered cubic
C)body-centered cubic
D)both face-centered and body-centered cubic
E)Simple, face-centered, and body-centered cubic all have the same packing efficiency.
A)simple cubic
B)face-centered cubic
C)body-centered cubic
D)both face-centered and body-centered cubic
E)Simple, face-centered, and body-centered cubic all have the same packing efficiency.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
Which unit cell contains the least atoms?
A)fcc
B)bcc
C)sc
D)both fcc and bcc
E)None of the above, as fcc, bcc, and sc contain the same number of atoms.
A)fcc
B)bcc
C)sc
D)both fcc and bcc
E)None of the above, as fcc, bcc, and sc contain the same number of atoms.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Polonium crystallizes in a simple cubic pattern. How many polonium atoms are in each unit cell?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
If a body-centered cubic unit cell has a volume of 1.447 108 pm3, what must be the dimension of the cube's edge?
A)1.131 108 pm
B)110 pm
C)1.20 104 pm
D)525 pm
E)367 pm
A)1.131 108 pm
B)110 pm
C)1.20 104 pm
D)525 pm
E)367 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
An atom in a particular unit cell has 6 nearest neighbor atoms around it. Which unit cell(s) are possible?
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
Compare the packing efficiency of face-centered cubic gold and face-centered cubic copper.
A)The efficiency of packing in the gold unit cell is higher.
B)The efficiency of packing in the copper unit cell is higher.
C)The efficiencies of packing in the unit cells of these two metals are the same.
D)Packing efficiencies are not defined for metals.
E)There is no way to compare without further information.
A)The efficiency of packing in the gold unit cell is higher.
B)The efficiency of packing in the copper unit cell is higher.
C)The efficiencies of packing in the unit cells of these two metals are the same.
D)Packing efficiencies are not defined for metals.
E)There is no way to compare without further information.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
In the cesium chloride unit cell shown below, the cesium ions sit on the corners of a cube. What is the name of this unit cell? 
A)simple cubic
B)chloride-centered cubic
C)face-centered cubic
D)cubic-centered
E)body-centered cubic

A)simple cubic
B)chloride-centered cubic
C)face-centered cubic
D)cubic-centered
E)body-centered cubic

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
An atom in a particular unit cell has 8 nearest neighbor atoms around it. Which unit cell(s) are possible?
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal
A)simple cubic
B)body-centered cubic
C)face-centered cubic
D)hexagonal
E)face-centered cubic or hexagonal

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
How many nearest neighbor atoms are there around each atom in a body-centered cubic unit cell?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
Compare the packing efficiency of face-centered cubic gold and face-centered cubic sodium chloride considering all the atoms.
A)The efficiency of packing in the gold unit cell is higher.
B)The efficiency of packing in the sodium chloride unit cell is higher.
C)The efficiencies of packing in the two lattices are the same.
D)Packing efficiencies cannot be defined for one or both of these.
E)There is no way to compare without further information.
A)The efficiency of packing in the gold unit cell is higher.
B)The efficiency of packing in the sodium chloride unit cell is higher.
C)The efficiencies of packing in the two lattices are the same.
D)Packing efficiencies cannot be defined for one or both of these.
E)There is no way to compare without further information.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
How many nearest neighbor atoms are there around each atom in a face-centered cubic unit cell?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
How many nearest neighbor atoms are there around each atom in a simple cubic unit cell?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Which unit cell contains the most atoms?
A)fcc
B)bcc
C)cubic
D)both fcc and bcc
E)None of the above, as fcc, bcc, and cubic contain the same number of atoms.
A)fcc
B)bcc
C)cubic
D)both fcc and bcc
E)None of the above, as fcc, bcc, and cubic contain the same number of atoms.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
Nickel (Ni) crystallizes as a face-centered unit cell with an edge length of 382 pm. What is the atomic radius of nickel?
A)135 pm
B)165 pm
C)191 pm
D)252 pm
E)1,080 pm
A)135 pm
B)165 pm
C)191 pm
D)252 pm
E)1,080 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
The higher the carbon content in steel, ________
A)the stronger and more malleable it is.
B)the stronger and more brittle it is.
C)the weaker and more malleable it is.
D)the weaker and more brittle it is.
E)Any of these, depending on the formula of the interstitial compound.
A)the stronger and more malleable it is.
B)the stronger and more brittle it is.
C)the weaker and more malleable it is.
D)the weaker and more brittle it is.
E)Any of these, depending on the formula of the interstitial compound.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement regarding the formation of an alloy is correct?
A)Carbon forms an interstitial alloy with iron because their atomic radii are about the same.
B)Carbon forms a substitutional alloy with iron because their atomic radii are about the same.
C)Carbon forms an interstitial alloy with iron because carbon has a much smaller atomic radius.
D)Carbon forms a substitutional alloy with iron because carbon has a much smaller atomic radius.
E)Carbon forms an interstitial alloy with iron because carbon has a much larger atomic radius.
A)Carbon forms an interstitial alloy with iron because their atomic radii are about the same.
B)Carbon forms a substitutional alloy with iron because their atomic radii are about the same.
C)Carbon forms an interstitial alloy with iron because carbon has a much smaller atomic radius.
D)Carbon forms a substitutional alloy with iron because carbon has a much smaller atomic radius.
E)Carbon forms an interstitial alloy with iron because carbon has a much larger atomic radius.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
If a face-centered cubic unit cell has a volume of 1.447 108 pm3 and the ions at the corners touch the ion on the face, what must be the ion's radius?
A)186 pm
B)388 pm
C)4,243 pm
D)125 pm
E)1,050 pm
A)186 pm
B)388 pm
C)4,243 pm
D)125 pm
E)1,050 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
A tetrahedral hole in a crystal lattice is defined as ________
A)one-half of an octahedral hole.
B)the space between any number of atoms having tetrahedral edges.
C)the space between a cage of sp3 hybridized atoms, such as in diamond.
D)the space between a cluster of four adjacent atoms arranged in a tetrahedron.
E)a large hole having four flat sides arranged in a tetrahedral shape.
A)one-half of an octahedral hole.
B)the space between any number of atoms having tetrahedral edges.
C)the space between a cage of sp3 hybridized atoms, such as in diamond.
D)the space between a cluster of four adjacent atoms arranged in a tetrahedron.
E)a large hole having four flat sides arranged in a tetrahedral shape.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following refers to an alloy in which the composition of the elements is variable and one element must have a much smaller radius than the other?
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)inhomogeneous
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)inhomogeneous

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
Iron (Fe) has a density of 7.874 g/cm3 and crystallizes in a body-centered cubic structure. What is the atomic radius of iron?
A)99 pm
B)114 pm
C)124 pm
D)143 pm
E)255 pm
A)99 pm
B)114 pm
C)124 pm
D)143 pm
E)255 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
The alpha form of polonium (Po) has a density of 9.196 g/cm3 and crystallizes in a simple cubic structure. What is the atomic radius of polonium?
A)119 pm
B)168 pm
C)266 pm
D)335 pm
E)419 pm
A)119 pm
B)168 pm
C)266 pm
D)335 pm
E)419 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
In addition to carbon and iron, stainless steel contains ________
A)Teflon and polyethylene.
B)gold and silver.
C)copper and nickel.
D)chromium and nickel.
E)platinum.
A)Teflon and polyethylene.
B)gold and silver.
C)copper and nickel.
D)chromium and nickel.
E)platinum.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following refers to an alloy in which the composition is variable and the elements have comparable radii?
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)homogeneous
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)homogeneous

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
Aluminum (Al) has a density of 2.70 g/cm3 and crystallizes in a face-centered cubic structure. What is the unit cell edge length?
A)2.47 10-3 pm
B)40.0 pm
C)405 pm
D)321 pm
E)255 pm
A)2.47 10-3 pm
B)40.0 pm
C)405 pm
D)321 pm
E)255 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
Platinum (Pt) has a face-centered cubic structure with an edge length of 135 pm. What is the calculated value of the density of platinum based on this information?
A)1.03 g/cm3
B)5.81 g/cm3
C)11.63 g/cm3
D)173 g/cm3
E)527 g/cm3
A)1.03 g/cm3
B)5.81 g/cm3
C)11.63 g/cm3
D)173 g/cm3
E)527 g/cm3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following refers to an alloy in which the composition of the elements is constant?
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)homogeneous
A)intermetallic
B)interstitial
C)stoichiometric
D)substitutional
E)homogeneous

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
The alpha form of polonium (Po) crystallizes as a simple cubic unit cell with an edge length of 335 pm. What is the atomic radius of polonium?
A)84 pm
B)168 pm
C)335 pm
D)175 pm
E)808 pm
A)84 pm
B)168 pm
C)335 pm
D)175 pm
E)808 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
Iron (Fe) crystallizes as a body-centered unit cell and has an atomic radius of 126 pm. What is the edge length for the unit cell of iron?
A)55 pm
B)63 pm
C)252 pm
D)291 pm
E)356 pm
A)55 pm
B)63 pm
C)252 pm
D)291 pm
E)356 pm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Bronze that is composed of 10% tin and 90% copper is ________
A)a substitutional alloy.
B)an interstitial alloy.
C)a doped semiconductor.
D)a colloidal alloy.
E)an intermetallic compound.
A)a substitutional alloy.
B)an interstitial alloy.
C)a doped semiconductor.
D)a colloidal alloy.
E)an intermetallic compound.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
An octahedral hole in a crystal lattice is defined as ________
A)twice the size of a tetrahedral hole.
B)the space between any number of atoms having octahedral edges.
C)the space between a cluster of six adjacent atoms arranged in a octahedron.
D)the space between layers of sp2 hybridized atoms, such as in graphite.
E)a large hole having six sides arranged in an octahedral shape.
A)twice the size of a tetrahedral hole.
B)the space between any number of atoms having octahedral edges.
C)the space between a cluster of six adjacent atoms arranged in a octahedron.
D)the space between layers of sp2 hybridized atoms, such as in graphite.
E)a large hole having six sides arranged in an octahedral shape.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
Gold has a face-centered cubic structure with a unit cell edge length of 407.8 pm. What is the contribution to the density from each individual gold atom?
A)21.44 g/cm3
B)26.20 g/cm3
C)13.1 g/cm3
D)6.55 g/cm3
E)19.28 g/cm3
A)21.44 g/cm3
B)26.20 g/cm3
C)13.1 g/cm3
D)6.55 g/cm3
E)19.28 g/cm3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following can be varied to change the physical properties of an alloy?
I.the elements used
II.the proportions used
III.the type of hole each element occupies
A)I only
B)II only
C)III only
D)I and II only
E)I, II, and III
I.the elements used
II.the proportions used
III.the type of hole each element occupies
A)I only
B)II only
C)III only
D)I and II only
E)I, II, and III

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
In a two-component alloy the more abundant metal can be thought of as the solvent while the less abundant metal can be thought of as the solute. Which of the following would not change the position of atoms in the solvent's unit cell?
I.a solute with the same atomic radius as the solvent
II.a solute that was sufficiently small to fit into holes in the solvent's unit cell
III.a solvent that was sufficiently small to fit into holes in the solute's unit cell
A)I only
B)II only
C)III only
D)I or II
E)I, II, or III
I.a solute with the same atomic radius as the solvent
II.a solute that was sufficiently small to fit into holes in the solvent's unit cell
III.a solvent that was sufficiently small to fit into holes in the solute's unit cell
A)I only
B)II only
C)III only
D)I or II
E)I, II, or III

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
A face-centered cubic unit cell contains ________ octahedral holes.
A)1
B)4
C)8
D)12
E)13
A)1
B)4
C)8
D)12
E)13

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
In fluorite, ________
A)cations are smaller than the anions, and there are two cations for every anion.
B)anions are smaller than the cations, and there are two anions for every cation.
C)cations are smaller than the anions, and there are two anions for every cation.
D)anions are smaller than the cations, and there are two cations for every anion.
E)anions and cations are the same size.
A)cations are smaller than the anions, and there are two cations for every anion.
B)anions are smaller than the cations, and there are two anions for every cation.
C)cations are smaller than the anions, and there are two anions for every cation.
D)anions are smaller than the cations, and there are two cations for every anion.
E)anions and cations are the same size.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
In antifluorite structures, ________
A)cations are smaller than the anions, and there are two cations for every anion.
B)anions are smaller than the cations, and there are two anions for every cation.
C)cations are smaller than the anions, and there are two anions for every cation.
D)anions are smaller than the cations, and there are two cations for every anion.
E)anions and cations are the same size.
A)cations are smaller than the anions, and there are two cations for every anion.
B)anions are smaller than the cations, and there are two anions for every cation.
C)cations are smaller than the anions, and there are two anions for every cation.
D)anions are smaller than the cations, and there are two cations for every anion.
E)anions and cations are the same size.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
The face-centered cubic unit cell has ________ tetrahedral holes.
A)0
B)1
C)4
D)8
E)12
A)0
B)1
C)4
D)8
E)12

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
What is the likely unit cell for ionic compounds of 1:1 stoichiometry in which the difference in the radii is only about 25% or less?
A)rock salt
B)sphalerite
C)fluorite
D)cubic
E)antifluorite
A)rock salt
B)sphalerite
C)fluorite
D)cubic
E)antifluorite

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
In comparing the density of bronze composed of 20% tin to the density of pure copper, ________
A)the density of the bronze is higher.
B)the density of the bronze is lower.
C)the density of the bronze is the same.
D)the density of the bronze depends on whether the tin or the copper occupies holes.
E)It cannot be determined, because only the 1:1 intermetallic compound of tin and copper has ever been observed.
A)the density of the bronze is higher.
B)the density of the bronze is lower.
C)the density of the bronze is the same.
D)the density of the bronze depends on whether the tin or the copper occupies holes.
E)It cannot be determined, because only the 1:1 intermetallic compound of tin and copper has ever been observed.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
Stainless steel is less susceptible to rusting than iron because ________
A)it is coated with plastic.
B)the metals other than iron in the alloy form protective oxides.
C)the carbon within the alloy polymerizes to form a protective film.
D)the silicon within the alloy oxidizes to form a protective silicate layer.
E)the intermetallic compound formed is less reactive.
A)it is coated with plastic.
B)the metals other than iron in the alloy form protective oxides.
C)the carbon within the alloy polymerizes to form a protective film.
D)the silicon within the alloy oxidizes to form a protective silicate layer.
E)the intermetallic compound formed is less reactive.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
What type of hole is depicted below? The gray circle represents the top layer. 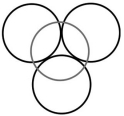
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
What type of hole is depicted below? The gray circles represent the top layer. 
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
In interstitial alloys, a maximum ( 1) atomic radius ratio would indicate an atom would likely fit in what type of hole(s)?
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
In the cubic closest-packed structure of sodium chloride, which ions are touching or nearly touching?
I.sodium ions and sodium ions
II.chloride ions and chloride ions
III.sodium ions and chloride ions
A)I only
B)II only
C)III only
D)II and III only
E)I and III only
I.sodium ions and sodium ions
II.chloride ions and chloride ions
III.sodium ions and chloride ions
A)I only
B)II only
C)III only
D)II and III only
E)I and III only

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
If half of the tetrahedral holes in a face-centered cubic unit cell are filled, what must be the stoichiometry of the ionic compound, written as nonhole sites:hole sites in lowest terms?
A)2:1
B)1:1
C)1:2
D)4:1
E)2:3
A)2:1
B)1:1
C)1:2
D)4:1
E)2:3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
The center of a cubic hole is found ________
A)at the center of a simple cubic lattice.
B)on the edges of a simple cubic lattice.
C)on the faces of a body-centered cubic lattice.
D)at the center of a face-centered cubic lattice.
E)at eight sites within a face-centered cubic lattice.
A)at the center of a simple cubic lattice.
B)on the edges of a simple cubic lattice.
C)on the faces of a body-centered cubic lattice.
D)at the center of a face-centered cubic lattice.
E)at eight sites within a face-centered cubic lattice.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
In interstitial alloys, a small ( 0.3) atomic radius ratio would indicate an atom would fit in what type of hole(s)?
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole
A)tetrahedral hole
B)octahedral hole
C)cubic hole
D)both an octahedral and tetrahedral hole
E)both an octahedral and cubic hole

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
Aluminum alloys are more desirable than steel in some applications because of their relatively ________
A)low density.
B)low cost.
C)high luster.
D)high warmth to touch.
E)high conductivity.
A)low density.
B)low cost.
C)high luster.
D)high warmth to touch.
E)high conductivity.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
Aluminum is resistant to corrosion because of ________
A)its positive oxidation potential.
B)its low density.
C)the formation of a protective surface film of aluminum oxide.
D)the formation of a protective surface film of aluminum nitride.
E)its lack of reactivity toward oxygen.
A)its positive oxidation potential.
B)its low density.
C)the formation of a protective surface film of aluminum oxide.
D)the formation of a protective surface film of aluminum nitride.
E)its lack of reactivity toward oxygen.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
If a salt has a face-centered cubic (fcc) unit cell and a 1:2 cation:anion stoichiometry with cations occupying the fcc positions, then the anions fill all the ________ holes.
A)Cubic
B)octahedral
C)tetrahedral
D)octahedral and tetrahedral
E)cubic, octahedral, and tetrahedral
A)Cubic
B)octahedral
C)tetrahedral
D)octahedral and tetrahedral
E)cubic, octahedral, and tetrahedral

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
A face-centered cubic unit cell has a(n) ________ in its center.
A)tetrahedral hole
B)octahedral hole
C)Atom
D)square planar hole
E)cubic hole
A)tetrahedral hole
B)octahedral hole
C)Atom
D)square planar hole
E)cubic hole

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
Why does a pure metal not crystallize in a fluorite or antifluorite unit cell?
A)They can and do.
B)These unit cells require two types of atoms or ions with differing radii.
C)Pure metals do not crystallize; they are amorphous.
D)The atoms in pure metals move about in a sea of electrons.
E)The radius of the metal is too large.
A)They can and do.
B)These unit cells require two types of atoms or ions with differing radii.
C)Pure metals do not crystallize; they are amorphous.
D)The atoms in pure metals move about in a sea of electrons.
E)The radius of the metal is too large.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
A rock salt structure has smaller ions in ________
A)cubic holes.
B)tetrahedral holes.
C)hexagonal holes.
D)octahedral holes.
E)the usual atomic positions in the unit cell, i.e., not in holes.
A)cubic holes.
B)tetrahedral holes.
C)hexagonal holes.
D)octahedral holes.
E)the usual atomic positions in the unit cell, i.e., not in holes.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck


